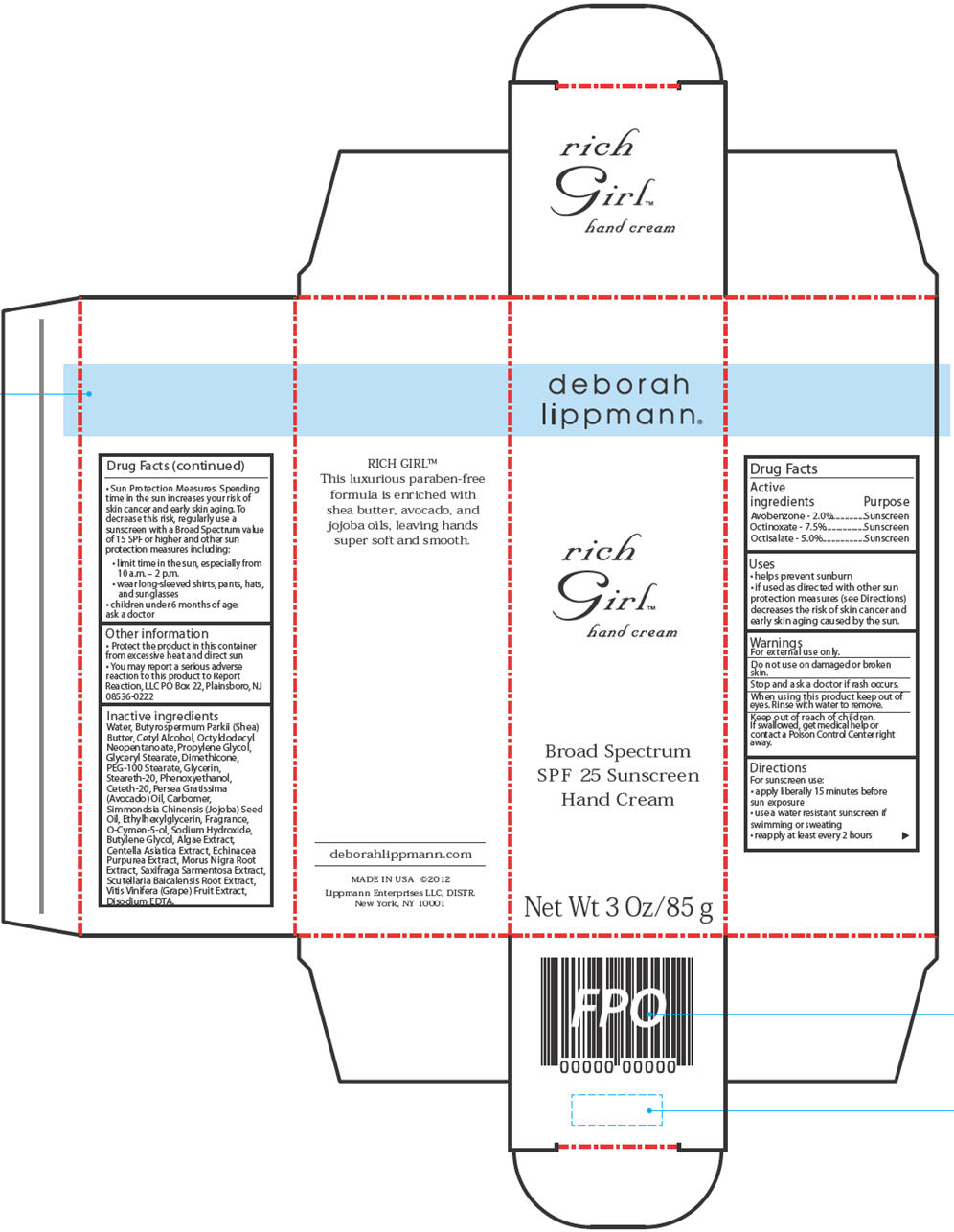
Label: RICH GIRL HAND SPF 25- rich girl hand cream
- NDC Code(s): 59735-420-01
- Packager: MANA PRODUCTS, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
-
DIRECTIONS
For sunscreen use:
apply liberally 15 minutes before sun exposure
use a water resistant sunscreen if swimming or sweating
reapply at least every 2 hours
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrumvalue of 15 SPF or higher and other sun protection measures including:
limit time in the sun, especially from 10 a.m. – 2 p.m.
wear long-sleeved shirts, pants, hats, and sunglasses
children under 6 months of age: ask a doctor - OTHER INFORMATION
-
INACTIVE INGREDIENTS
Water, Butyrospermum Parkii (Shea) Butter, Cetyl Alcohol, Octyldodecyl Neopentanoate, Propylene Glycol, Glyceryl Stearate, Dimethicone, PEG-100 Stearate, Glycerin, Steareth-20,Phenoxyethanol, Ceteth-20, Persea Gratissima (Avocado) Oil, Carbomer, Simmondsia Chinensis (Jojoba) Seed Oil, Ethylhexylglycerin, Fragrance, O-Cymen-5-ol, Sodium Hydroxide,Butylene Glycol, Algae Extract, Centella Asiatica Extract, Echinacea Purpurea Extract, Morus Nigra Root Extract, Saxifraga Sarmentosa Extract, Scutellaria Baicalensis Root Extract,Vitis Vinifera (Grape) Fruit Extract, Disodium EDTA.
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- LABEL
-
INGREDIENTS AND APPEARANCE
RICH GIRL HAND SPF 25
rich girl hand creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59735-420 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIMETHICONE (UNII: 92RU3N3Y1O) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) AVOCADO OIL (UNII: 6VNO72PFC1) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CENTELLA ASIATICA (UNII: 7M867G6T1U) ECHINACEA PURPUREA (UNII: QI7G114Y98) SAXIFRAGA STOLONIFERA LEAF (UNII: O3TMV4903H) WINE GRAPE (UNII: 3GOV20705G) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) SHEA BUTTER (UNII: K49155WL9Y) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) STEARETH-20 (UNII: L0Q8IK9E08) CETETH-20 (UNII: I835H2IHHX) JOJOBA OIL (UNII: 724GKU717M) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) O-CYMEN-5-OL (UNII: H41B6Q1I9L) SODIUM HYDROXIDE (UNII: 55X04QC32I) MORUS NIGRA ROOT (UNII: 033456LFG1) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59735-420-01 1 in 1 CARTON 05/15/2012 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/15/2012 Labeler - MANA PRODUCTS, INC. (078870292) Establishment Name Address ID/FEI Business Operations MANA PRODUCTS, INC 032870270 manufacture(59735-420) Establishment Name Address ID/FEI Business Operations MANA PRODUCTS, INC. 078870292 manufacture(59735-420)