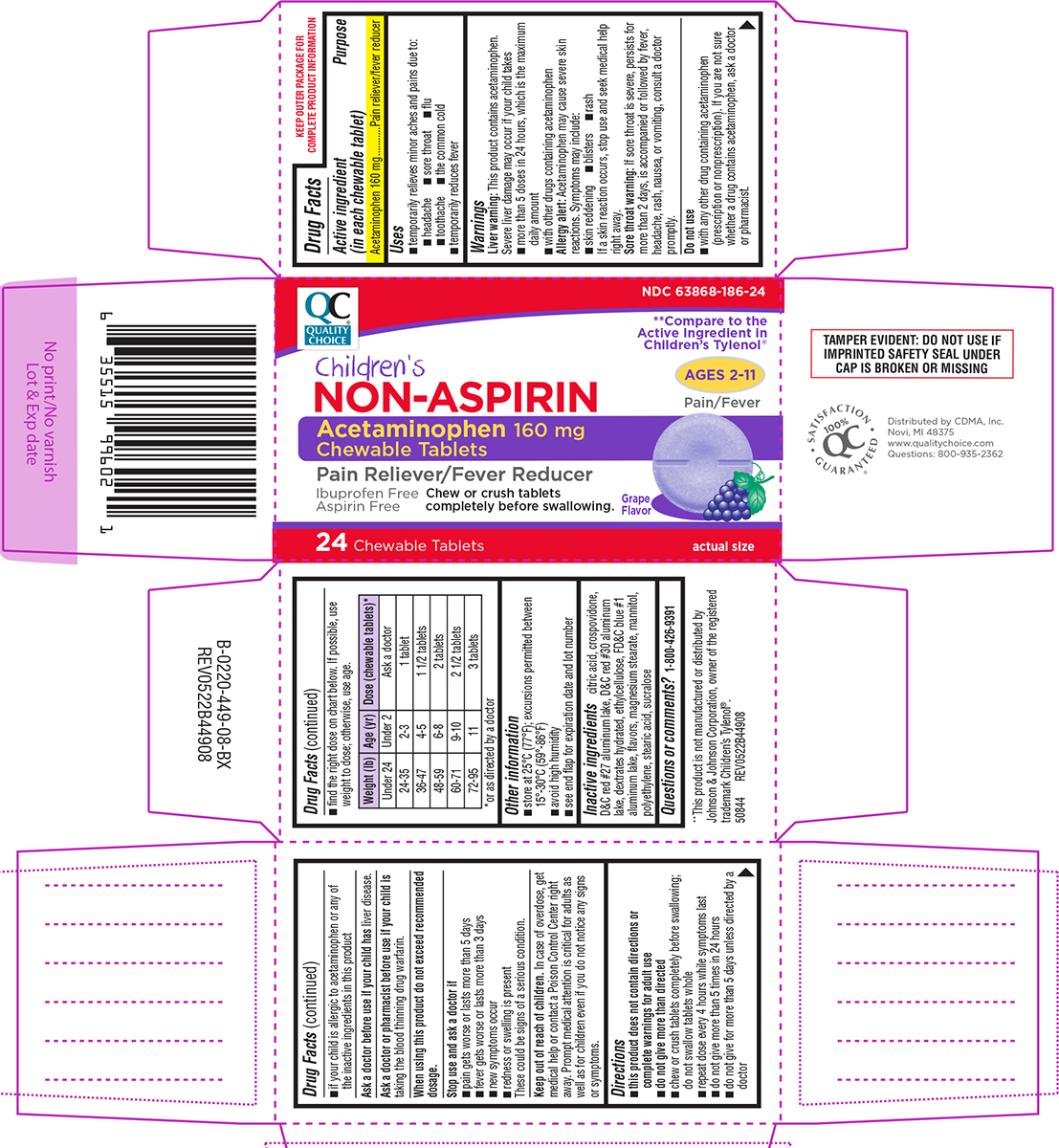
Label: NON-ASPIRIN CHILDRENS- acetaminophen tablet, chewable
- NDC Code(s): 63868-186-24
- Packager: CHAIN DRUG MARKETING ASSOCIATION INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
-
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
Weight (lb) Age (yr)
Dose (chewable tablets)*
Under 24
Under 2
Ask a doctor
24-35
2-3
1 tablet
36-47
4-5
1 1/2 tablets
48-59
6-8
2 tablets
60-71
9-10
2 1/2 tablets
72-95
11
3 tablets
*or as directed by a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
QC®
QUALITY
CHOICENDC 63868-186-24
**Compare to the
Active Ingredient in
Children’s Tylenol®Children's
NON-ASPIRINAcetaminophen 160 mg
Chewable TabletsPain Reliever / Fever Reducer
Ages 2-11
Pain/FeverIbuprofen Free
Aspirin FreeChew or crush tablets
completely before swallowing.Grape
Flavoractual size
24 CHEWABLE TABLETS
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER
CAP IS BROKEN OR MISSINGDistributed by CDMA, Inc.
Novi, MI 48375
www.qualitychoice.com
Questions: 800-935-2362SATISFACTION GUARANTEED
100% QC®**This product is not manufactured or distributed by
Johnson & Johnson Corporation, owner of the registered
trademark Children’s Tylenol®.
50844 REV0522B44908
Quality Choice 44-449
-
INGREDIENTS AND APPEARANCE
NON-ASPIRIN CHILDRENS
acetaminophen tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-186 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color purple Score 2 pieces Shape ROUND Size 16mm Flavor GRAPE Imprint Code 44;449 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-186-24 1 in 1 CARTON 01/28/2005 1 24 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 01/28/2005 Labeler - CHAIN DRUG MARKETING ASSOCIATION INC (011920774) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(63868-186) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(63868-186) , pack(63868-186) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(63868-186) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(63868-186)