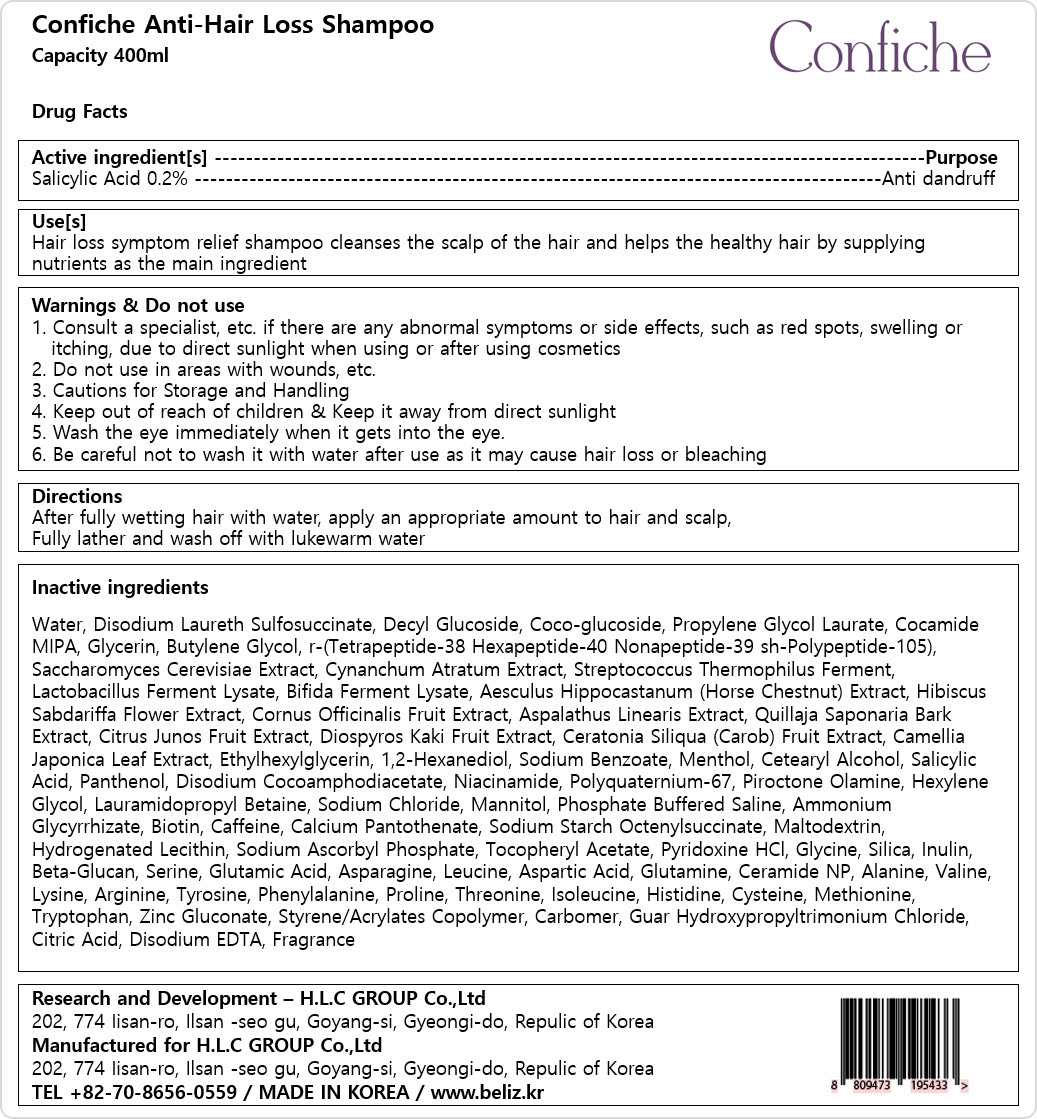
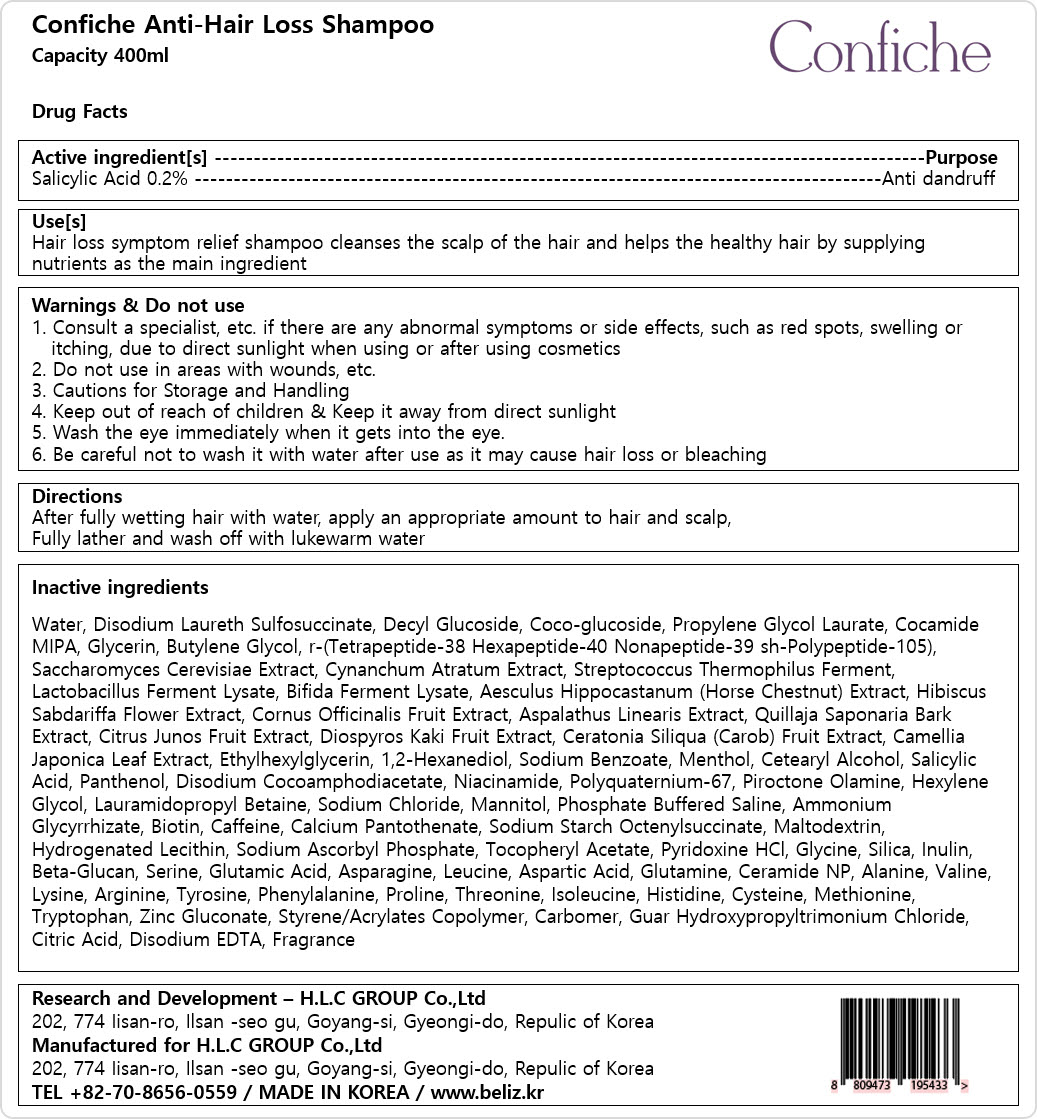
Label: CONFICHE ANTI-HAIR LOSSSHAMPOO- salicylic acid shampoo
- NDC Code(s): 81555-301-01
- Packager: H.L.C GROUP Co., ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
1. Consult a specialist, etc. if there are any abnormal symptoms or side effects, such as red spots, swelling or
itching, due to direct sunlight when using or after using cosmetics
2. Do not use in areas with wounds, etc.
3. Cautions for Storage and Handling
4. Keep out of reach of children & Keep it away from direct sunlight
5. Wash the eye immediately when it gets into the eye.
6. Be careful not to wash it with water after use as it may cause hair loss or bleaching
- Warnings
- Warnings
- Warnings
- Warnings
- Directions
-
Inactive ingredients
Water, Disodium Laureth Sulfosuccinate, Decyl Glucoside, Coco-glucoside, Propylene Glycol Laurate, Cocamide
MIPA, Glycerin, Butylene Glycol, r-(Tetrapeptide-38 Hexapeptide-40 Nonapeptide-39 sh-Polypeptide-105),
Saccharomyces Cerevisiae Extract, Cynanchum Atratum Extract, Streptococcus Thermophilus Ferment,
Lactobacillus Ferment Lysate, Bifida Ferment Lysate, Aesculus Hippocastanum (Horse Chestnut) Extract, Hibiscus
Sabdariffa Flower Extract, Cornus Officinalis Fruit Extract, Aspalathus Linearis Extract, Quillaja Saponaria Bark
Extract, Citrus Junos Fruit Extract, Diospyros Kaki Fruit Extract, Ceratonia Siliqua (Carob) Fruit Extract, Camellia
Japonica Leaf Extract, Ethylhexylglycerin, 1,2-Hexanediol, Sodium Benzoate, Menthol, Cetearyl Alcohol, Salicylic
Acid, Panthenol, Disodium Cocoamphodiacetate, Niacinamide, Polyquaternium-67, Piroctone Olamine, Hexylene
Glycol, Lauramidopropyl Betaine, Sodium Chloride, Mannitol, Phosphate Buffered Saline, Ammonium
Glycyrrhizate, Biotin, Caffeine, Calcium Pantothenate, Sodium Starch Octenylsuccinate, Maltodextrin,
Hydrogenated Lecithin, Sodium Ascorbyl Phosphate, Tocopheryl Acetate, Pyridoxine HCl, Glycine, Silica, Inulin,
Beta-Glucan, Serine, Glutamic Acid, Asparagine, Leucine, Aspartic Acid, Glutamine, Ceramide NP, Alanine, Valine,
Lysine, Arginine, Tyrosine, Phenylalanine, Proline, Threonine, Isoleucine, Histidine, Cysteine, Methionine,
Tryptophan, Zinc Gluconate, Styrene/Acrylates Copolymer, Carbomer, Guar Hydroxypropyltrimonium Chloride,
Citric Acid, Disodium EDTA, Fragrance
- Display Panel
-
INGREDIENTS AND APPEARANCE
CONFICHE ANTI-HAIR LOSSSHAMPOO
salicylic acid shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81555-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 mg in 100 mL Inactive Ingredients Ingredient Name Strength LEUCINE (UNII: GMW67QNF9C) ASPARTIC ACID (UNII: 30KYC7MIAI) GLUTAMINE (UNII: 0RH81L854J) CERAMIDE NP (UNII: 4370DF050B) VALINE (UNII: HG18B9YRS7) ARGININE (UNII: 94ZLA3W45F) THREONINE (UNII: 2ZD004190S) ISOLEUCINE (UNII: 04Y7590D77) GLYCERIN (UNII: PDC6A3C0OX) VINCETOXICUM ATRATUM WHOLE (UNII: U3176B0S5J) QUILLAJA SAPONARIA BARK (UNII: 8N0K3807ZW) CAROB (UNII: 5MG5Z946UO) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALANINE (UNII: OF5P57N2ZX) LYSINE (UNII: K3Z4F929H6) TYROSINE (UNII: 42HK56048U) PHENYLALANINE (UNII: 47E5O17Y3R) PROLINE (UNII: 9DLQ4CIU6V) HISTIDINE (UNII: 4QD397987E) CYSTEINE (UNII: K848JZ4886) METHIONINE (UNII: AE28F7PNPL) ZINC GLUCONATE (UNII: U6WSN5SQ1Z) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ESCHERICHIA COLI (UNII: 514B9K0L10) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) COCO GLUCOSIDE (UNII: ICS790225B) CITRUS JUNOS FRUIT (UNII: 53KHW58C1V) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) STREPTOCOCCUS THERMOPHILUS (UNII: T8F3MOB0CG) PERSIMMON (UNII: 4V023DD7KL) PIROCTONE OLAMINE (UNII: A4V5C6R9FB) MANNITOL (UNII: 3OWL53L36A) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LIMOSILACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) SODIUM CHLORIDE (UNII: 451W47IQ8X) GLYCINE (UNII: TE7660XO1C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRANSFORMING GROWTH FACTOR BETA RECEPTOR TYPE 3 (UNII: 18YWT2KYS8) SERINE (UNII: 452VLY9402) ASPARAGINE (UNII: 5Z33R5TKO7) MENTHOL (UNII: L7T10EIP3A) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURAMIDOPROPYL BETAINE (UNII: 23D6XVI233) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) GLUTAMIC ACID (UNII: 3KX376GY7L) BIFIDOBACTERIUM BIFIDUM R0071 (UNII: ZQ2KAH6G2Q) TETRAPEPTIDE-7 (UNII: X519T00027) DISODIUM LAURETH SULFOSUCCINATE (UNII: D6DH1DTN7E) COCO MONOISOPROPANOLAMIDE (UNII: 21X4Y0VTB1) SACCHAROMYCES CEREVISIAE (UNII: 978D8U419H) BIOTIN (UNII: 6SO6U10H04) CAFFEINE (UNII: 3G6A5W338E) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INULIN (UNII: JOS53KRJ01) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL MONOLAURATE (UNII: 668Z5835Z3) HORSE CHESTNUT (UNII: 3C18L6RJAZ) HIBISCUS SABDARIFFA FLOWER (UNII: 45TGG6IU6M) CORNUS OFFICINALIS FRUIT (UNII: 23NL8NQ187) ASPALATHUS LINEARIS LEAF (UNII: H7UGK1GJCU) CAMELLIA JAPONICA LEAF (UNII: 4E3VE6KTLY) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM BENZOATE (UNII: OJ245FE5EU) PANTHENOL (UNII: WV9CM0O67Z) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) NIACINAMIDE (UNII: 25X51I8RD4) MALTODEXTRIN (UNII: 7CVR7L4A2D) HEXYLENE GLYCOL (UNII: KEH0A3F75J) PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) TRYPTOPHAN (UNII: 8DUH1N11BX) STYRENE/ACRYLAMIDE COPOLYMER (MW 500000) (UNII: 5Z4DPO246A) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (1.7 SUBSTITUENTS PER SACCHARIDE) (UNII: B16G315W7A) FRAGRANCE 13576 (UNII: 5EM498GW35) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81555-301-01 400 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/01/2022 Labeler - H.L.C GROUP Co., ltd (694869128) Registrant - H.L.C GROUP Co., ltd (694869128) Establishment Name Address ID/FEI Business Operations H.L.C GROUP Co., ltd 695436080 manufacture(81555-301)