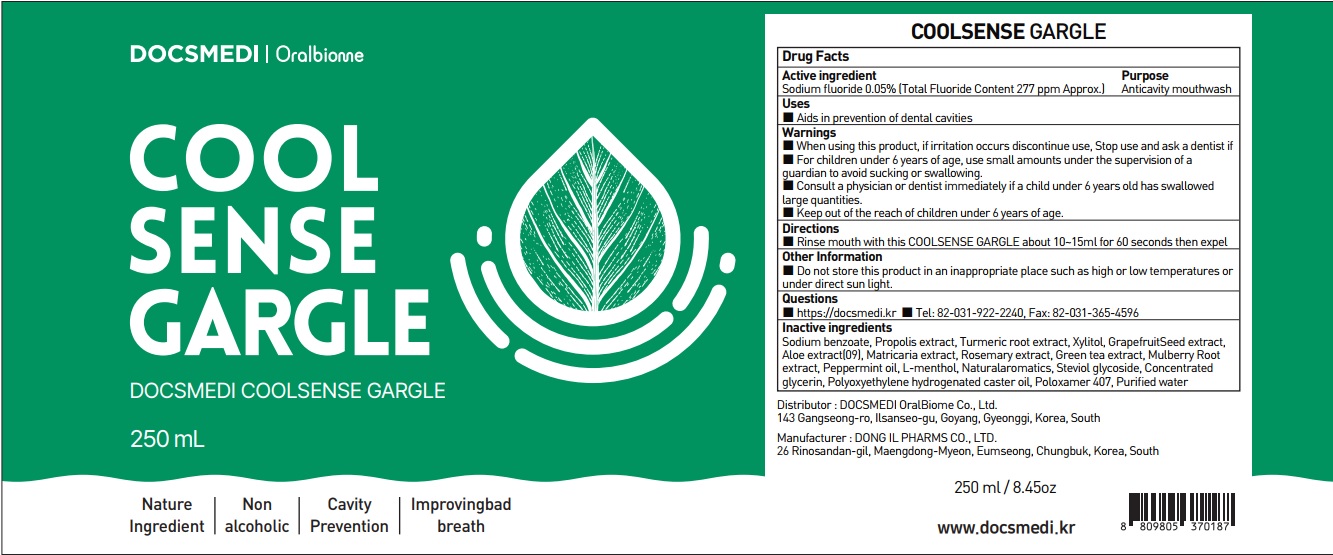
Label: COOL SENSE CARE PLUS- sodium fluoride mouthwash
- NDC Code(s): 76670-0013-1
- Packager: DOCSMEDI OralBiome Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
Inactive ingredients
Sodium benzoate, Propolis extract, Turmeric root extract, Xylitol, GrapefruitSeed extract, Aloe extract(09), Matricaria extract, rosemary extract, Green tea extract, Mulberry Root extract, Peppermint oil, L-menthol, Naturalaromatics, Steviol glycoside, Concentrated glycerin, Polyoxyethylene hydrogenated caster oil, Poloxamer 407, Purified water
- PURPOSE
-
WARNINGS
■ When using this product, if irritation occurs discontinue use, Stop use and ask a dentist if
■ For children under 6 years of age, use small amounts under the supervision of a guardian to avoid sucking or swallowing.
■ Consult a physician or dentist immediately if a child under 6 years old has swallowed large quantities.
■ Keep out of the reach of children under 6 years of age. - KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- Other Information
- Questions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COOL SENSE CARE PLUS
sodium fluoride mouthwashProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76670-0013 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium fluoride (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength Sodium benzoate (UNII: OJ245FE5EU) PROPOLIS WAX (UNII: 6Y8XYV2NOF) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76670-0013-1 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 12/01/2022 Labeler - DOCSMEDI OralBiome Co., Ltd. (694505169) Registrant - DOCSMEDI OralBiome Co., Ltd. (694505169) Establishment Name Address ID/FEI Business Operations DONG IL PHARMS CO., LTD. 557810721 manufacture(76670-0013)