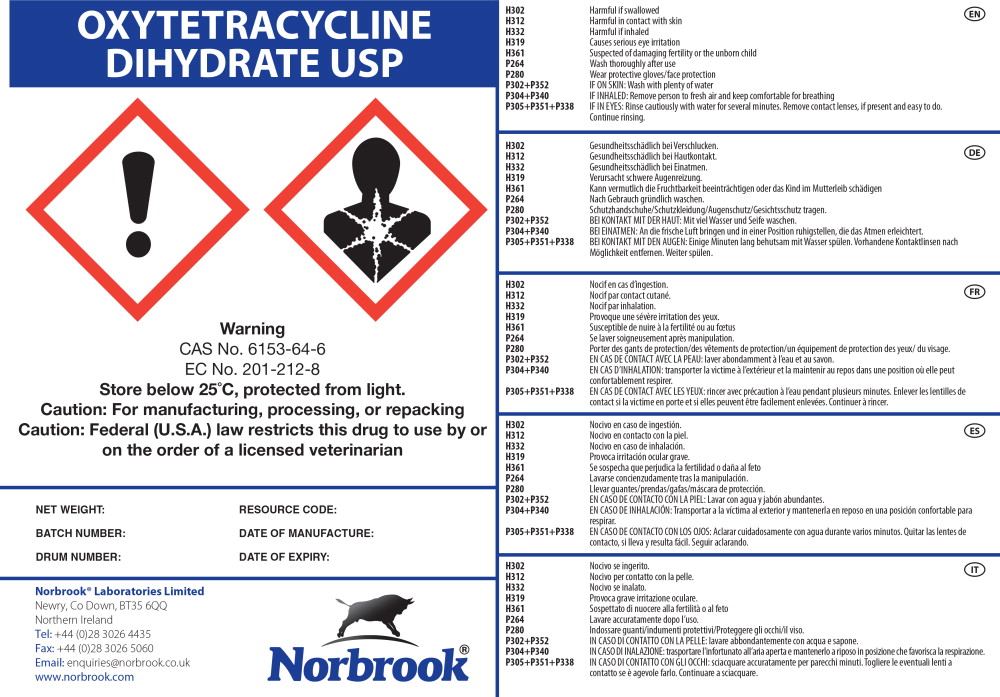
Label: OXYTETRACYCLINE DIHYDRATE- oxytetracycline powder
- NDC Code(s): 55529-650-32, 55529-650-33
- Packager: Norbrook Laboratories Limited
- Category: BULK INGREDIENT - ANIMAL DRUG
- DEA Schedule: None
- Marketing Status: bulk ingredient
Drug Label Information
Updated May 15, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Principal Display Panel - Drum Label
OXYTETRACYCLINE
DIHYDRATE USP
Warning
CAS No. 6153-64-6
EC No. 201-212-8
Store below 25°C, protected from light.
Caution: For manufacturing, processing, or repacking
Caution: Federal (U.S.A.) law restricts this drug to use by or
on the order of a licensed veterinarianNET WEIGHT: RESOURCE CODE:
BATCH NUMBER: DATE OF MANUFACTURE:
DRUM NUMBER: DATE OF EXPIRY:
Norbrook® Laboratories Limited
Newry, Co Down, BT35 6QQ
Northern Ireland
Tel: +44 (0)28 3026 4435
Fax: +44 (0)28 3026 5060
Email: enquiries@norbrook.co.uk
www.norbrook.com
Norbrook®
-
INGREDIENTS AND APPEARANCE
OXYTETRACYCLINE DIHYDRATE
oxytetracycline powderProduct Information Product Type Item Code (Source) NDC:55529-650 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Oxytetracycline (UNII: X20I9EN955) (Oxytetracycline Anhydrous - UNII:SLF0D9077S) Oxytetracycline 1 kg in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55529-650-32 10 kg in 1 DRUM 2 NDC:55529-650-33 25 kg in 1 DRUM Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date bulk ingredient 05/16/2006 Labeler - Norbrook Laboratories Limited (214580029) Establishment Name Address ID/FEI Business Operations Norbrook Laboratories Limited 232880554 API MANUFACTURE, ANALYSIS