Label: DIATRIZOATE MEGLUMINE AND DIATRIZOATE SODIUM solution
- NDC Code(s): 53041-688-03, 53041-690-03, 53041-690-09
- Packager: Guardian Drug Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 22, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Diatrizoate Meglumine and Diatrizoate Sodium Solution USP is a palatable lemon-flavored water-soluble iodinated radiopaque contrast medium for oral or rectal administration only. Each mL contains 660 mg diatrizoate meglumine and 100 mg diatrizoate sodium; pH has been adjusted to 6.0 to 7.6 with sodium hydroxide. Each mL contains approximately 4.8 mg (0.21 mEq) sodium and 367 mg organically bound iodine. Inactive ingredients: edetate disodium, flavor, hydrochloric acid, polysorbate 80, purified water, saccharin sodium, simethicone, sodium citrate and sodium hydroxide.
Diatrizoate meglumine is designated chemically as 1-deoxy-1-(methylamino)-D-glucitol 3,5-diacetamido-2,4,6-triiodo-benzoate (salt); diatrizoate sodium is monosodium 3, 5-diacetamido-2,4,6-triiodobenzoate. Structural formulas:
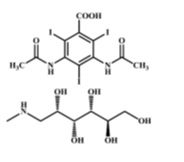
Diatrizoate Meglumine
C11H9I3N2O4.C7H17NO5
MW 809.13
Organically Bound Iodine: 47.1%
CAS – 131-49-7
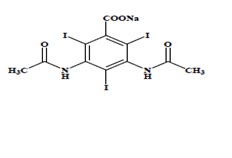
Diatrizoate Sodium
C11H8I3N2NaO4
MW 635.90
Organically Bound Iodine: 59.9%
CAS – 737-31-5 -
CLINICAL PHARMACOLOGY
The most important characteristic of contrast media is the iodine content. The relatively high atomic weight of iodine contributes sufficient radiodensity for radiographic contrast with surrounding tissues.
Diagnostic enteral radiopaque agents have few known pharmacological effects. Diatrizoate meglumine and diatrizoate sodium exert a mild laxative effect attributable to their high osmolarity.
Diatrizoate meglumine and diatrizoate sodium are sparingly absorbed from the intact gastrointestinal tract, and therefore permit gastrointestinal opacification and delineation after oral or rectal administration. Oral administration is used for radiographic evaluation of the esophagus, stomach and proximal small intestine. Rectal administration is used for examination of the colon; however, visualization of the distal small bowel is generally unsatisfactory, since the hypertonicity of the medium causes intraluminal diffusion of water with subsequent dilution of the medium. Enough absorption from the gastrointestinal tract to permit incidental visualization of urinary tract has been reported; this should be considered when thyroid testing is being contemplated, since iodine-mediated thyrotropic effects may occur (see PRECAUTIONS).
-
INDICATIONS & USAGE
Diatrizoate Meglumine and Diatrizoate Sodium Solution is indicated for radiographic examination of segments of the gastrointestinal tract (esophagus, stomach, proximal small intestine, and colon). The preparation is particularly indicated when a more viscous agent such as barium sulfate, which is not water-soluble, is not feasible or is potentially dangerous.
Diatrizoate Meglumine and Diatrizoate Sodium Solution may also be used as an adjunct to contrast enhancement is computed tomography of the torso (body imaging); the preparation is indicated, in conjunction with intravenous administration of a radiopaque contrast agent, when unenhanced imaging may not provide sufficient definition in distinguishing normal loops of bowel from adjacent organs or areas of suspected pathology.
- CONTRAINDICATIONS
-
WARNINGS
Dehydration: Administration of hypertonic Diatrizoate Meglumine and Diatrizoate Sodium Solution may lead to hypovolemia and hypotension due to fluid loss from the intestine. A 1 in 4.6 (1:4.6) dilution of Diatrizoate Meglumine and Diatrizoate Sodium Solution yields an approximately isotonic 16.5 percent diatrizoate salts solution; less dilute solutions are hypertonic and may lead to intraluminal movement of fluid with resulting hypovolemia. In young or debilitated children and in elderly cachectic persons, the loss of plasma fluid may be sufficient to cause a shock-like state. If Diatrizoate Meglumine and Diatrizoate Sodium Solution is used in infants and children (under 10 kg) or in dehydrated or debilitated patients, the solution must be prepared using the specific dilutions described in DOSAGE AND ADMINISTRATION . In debilitated patients and in patients with electrolyte imbalances, postprocedural monitoring of hydration, serum osmolarity, electrolytes and clinical status is essential. In pediatric or severely debilitated patients, the maintenance of an open intravenous fluid line for rehydration may be advisable should hypotension or shock supervene. Electrolyte disturbances must be corrected prior to the administration of any hypertonic Diatrizoate Meglumine and Diatrizoate Sodium Solution.
Aspiration: Aspiration of Diatrizoate Meglumine and Diatrizoate Sodium Solution into the trachea and airways may result in serious pulmonary complications including, pulmonary edema, pneumonitis or death Bronchial entry of any orally administered contrast medium causes a copious osmotic effusion. Therefore, avoid use of Diatrizoate Meglumine and Diatrizoate Sodium Solution in patients with esophagotracheal fistula and minimize risks for pulmonary aspiration in all patients. If Diatrizoate Meglumine and Diatrizoate Sodium Solution is given by nasogastric tube, the position of the tube in the stomach must be verified before administration.
Anaphylactic reactions: Anaphylactic reactions, including fatalities, have been reported with the use of Diatrizoate Meglumine and Diatrizoate Sodium Solution. Patients at increased risk include those with a history of a previous reaction to a contrast medium, patients with a known sensitivity to iodine, and patients with a known clinical hypersensitivity (bronchial asthma, hay fever, and food allergies). Medical personnel trained in the treatment of anaphylactic reactions and the necessary drugs and medical equipment should always be readily available when Diatrizoate Meglumine and Diatrizoate Sodium Solution is used.
-
PRECAUTIONS
GENERAL PRECAUTIONS
Diagnostic procedures which involve the use of radiopaque contrast agents should be carried out under the direction of personnel with the prerequisite training and with a thorough knowledge of the particular procedure to be performed. Appropriate facilities should be available for coping with any complication of administration, as well as for treatment of reaction to the contrast medium (see ADVERSE REACTIONS, and PRECAUTIONS,Information for the Patient).
Rectal administration of undiluted Diatrizoate Meglumine and Diatrizoate Sodium Solution in any patient, particularly with large doses and/or in those with overdistention, has been reported to be associated with mucosal irritation.
Cases of hyperthyroidism have been reported with the use of oral contrast media. Some of these patients reportedly had multinodular goiters which may have been responsible for the increased hormone synthesis in response to excess iodine. Administration of an intravascular iodinated radiopaque diagnostic agent to a hyperthyroid patient precipitated thyroid storm; a similar situation could follow administration of oral preparations of iodides. Therefore, caution should be exercised when administering enteral gastrointestinal radiopaque agents to hyperthyroid and euthyroid goiterous patients.
Consideration should be given to the potential for precipitation of water-soluble contrast agents under conditions that may promote hyperacidity (i.e., fasting, emotional upset, or stress). Harmful effects directly attributable to precipitate formation have not been reported. However, the possibility of interpreting the precipitate radiologically as an anatomical abnormality (i.e., ulceration of the stomach or small intestine) or injury, should be kept in mind.
INFORMATION FOR PATIENTS
Patients should receive the following information and instructions:
- This drug has been prescribed to perform an x-ray of the gastrointestinal tract.
- Inform the physician if pregnant or if allergic to iodine, any foods, or x-ray materials.
- The iodine in diatrizoate salts may interfere with some thyroid tests if these are needed in the future. Inform the attending physician at that time about this gastrointestinal study.
- This drug may cause abdominal cramping, nausea, vomiting, diarrhea, skin rashes, itching, heartburn, dizziness, or headache in some patients, but most reactions are mild and pass quickly
DRUG & OR LABORATORY TEST INTERACTIONS
Thyroid Function Tests
The results of protein bound iodine (PBI) and radioactive iodine uptake studies, which depend on iodine estimations, will not accurately reflect thyroid function for six months, and possibly as long as one year, following the administration of diagnostic enteral radiopaque media.
Thyroid function tests, if indicated, generally should be performed prior to the administration of any iodinated agent. However, thyroid function can be evaluated after use of these agents by using T3 resin uptake and total or free thyroxine (T4) assays which are not dependent on iodine estimations.
Pancreatic Tests
Small quantities of contrast medium in the intestinal tract may cause false low trypsin values when determined spectrophotometrically. Therefore, duodenal instillation should not precede pancreatic function tests involving spectrophotometric trypsin assays.
Any test which might be affected by contrast media should be performed prior to administration of the contrast medium.CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Long-term studies in animals have not been performed to evaluate carcinogenic or mutagenic potential, or possible impairment of fertility in males or females
PREGNANCY
When administered intravenously, diatrizoate salts cross the placenta and are evenly distributed in fetal tissues.
No teratogenic effects attributable to diatrizoate meglumine or diatrizoate sodium have been observed in teratology studies performed in animals. There are, however, no adequate and well-controlled studies in pregnant women. Because small amounts of these agents may be absorbed, and animal teratology studies are not always predictive of human response, these agents should be used during pregnancy only when clearly needed.
Procedures including radiation involve a certain risk related to the exposure of the fetus.
NURSING MOTHERS
Diatrizoate meglumine is excreted in breast milk following intravascular administration.
Because small amounts of enteral gastrointestinal radiopaque agents may be absorbed following oral or rectal administration, caution should be exercised when they are administered to a nursing woman.
PEDIATRIC USE
See WARNINGS, and PRECAUTIONS, General
Local injury to the colonic mucosa, particularly in the presence of underlying disease which interferes with intestinal viability, has been reported in cases where recommended doses and dilutions (see DOSAGE AND ADMINISTRATION ) were not used; when extemporaneous dosage is elected, the polysorbate 80 level in the dose may be contributing factor to injury. -
ADVERSE REACTIONS
Most adverse reactions to enteral diagnostic radiopaque agents are mild and transitory. Nausea, vomiting and/or diarrhea, urticaria with erythema, hypoxia, acute dyspnea, tachyarrhythmia, and anaphylaxis have occurred following ingestion of the contrast medium, particularly when high concentrations of large volumes of solution are administered. Severe changes in serum osmolarity and electrolyte concentrations may produce shock-like states (see WARNINGS). It should be kept in mind that serious or anaphylactoid reactions that may occur with intravascular administration of radiopaque contrast agents are theoretically possible following administration by other routes.
-
OVERDOSAGE
See WARNINGS regarding potential hypovolemia, hypotension, or shock. The maintenance of an open intravenous fluid line for rehydration may be advisable. See DOSAGE AND ADMINISTRATION for appropriate doses and dilutions. Treatment of an overdose should be directed toward the support of all vital functions, and prompt institution of symptomatic therapy.
-
DOSAGE & ADMINISTRATION
General
This medium is not to be used for the preparations of solutions for parenteral administration. Oral or rectal administration only. Discard any unused portion after procedure.
The routine preparatory measures employed for barium studies are also appropriate for this agent.
For pediatric and severely cachectic patients the maintenance of an intravenous fluid line may be advisable.
Radiographic Examination of Segments of the Gastrointestinal Tract
Oral Administration: Adult oral dosage may range from 30 to 90 mL (11 to 33 g iodine), depending on the nature of the examination and the size of the patient. For infants and children less than 5 years of age, 30 mL (11 g iodine) are usually adequate; for children 5 to 10 years of age, the suggested dose is 60 mL (22 g iodine). These pediatric doses may be diluted 1:1, if desired, with water, carbonated beverage, milk or mineral oil. When used in infants, the solution may be given in a nursing bottle. Pediatric doses may also be used in dehydrated and/or debilitated adult patients. A 1:1 dilution is also recommended when the contrast medium is used in elderly cachectic individuals.
For very young (under 10 kg) and debilitated children the dose should be diluted: 1 part Diatrizoate Meglumine and Diatrizoate Sodium Solution in 3 parts water is recommended.
For Enemas or Enterostomy Instillations: Diatrizoate Meglumine and Diatrizoate Sodium Solution should be diluted when it is used for enemas and enterostomy instillations. When used as an enema, the suggested dilution for adults is 240 mL (88 g iodine) in 1,000 mL of tap water. For children under 5 years of age, a 1:5 dilution in tap water is suggested; for children over 5 years of age, 90 mL (33 g iodine) in 500 mL of tap water is a suitable dilution.
Tomography (Body Imaging)
A usual adult dose is 240 mL of a dilute Diatrizoate Meglumine and Diatrizoate Sodium Solution prepared by diluting 25 mL (9.17 g iodine) to one liter with tap water. Less dilute solutions [up to 77 mL (28.26 g iodine) diluted to one liter with tap water] may be used when indicated. The dose is administered orally about 15 to 30 minutes prior to imaging in order to permit the contrast medium to reach the pelvic loops.
-
HOW SUPPLIED
Diatrizoate Meglumine and Diatrizoate Sodium Solution USP is a clear, colorless to pale yellow liquid with citrus aroma and is available in packages of:
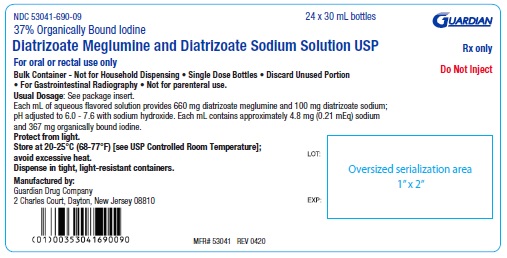
NDC 53041-690-09 Twenty-four 30 mL single dose bottles
NDC 53041-690-03 Twelve 120 mL single dose bottles
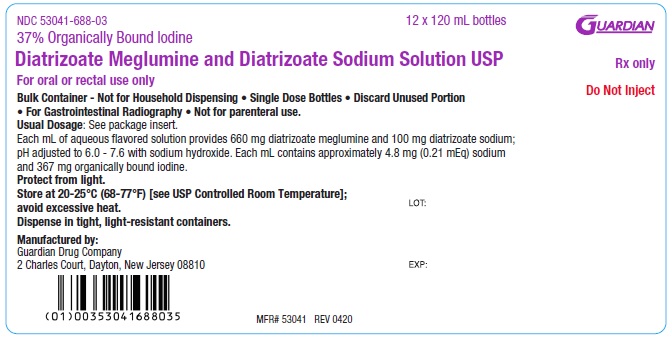
NDC 53041-688-03 Twelve 120 mL single dose bottles (PET)
Storage
Protect from light. Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature]; avoid excessive heat. Discard unused portion.
Manufactured by:
Guardian Drug Company
2 Charles Court, Dayton, New Jersey 08810
Revised: 08/2020
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
37% Organically Bound Iodine
Diatrizoate Meglumine and Diatrizoate Sodium Solution USP
For oral or rectal use only
Do Not Inject
Rx only
30 mL
Guardian

NDC 53041-690-09
24 x 30 mL Bottle Shipper/case label
Diatrizoate Meglumine and Diatrizoate Sodium Solution USP
NDC 53041-690-03
37% Organically Bound Iodine
Diatrizoate Meglumine and Diatrizoate Sodium Solution USP
For oral or rectal use only
Do Not Inject
Rx only
120 mL
Guardian

NDC 53041-690-03
12 x 120 mL Bottle Shipper/case label
Diatrizoate Meglumine and Diatrizoate Sodium Solution USP

NDC 53041-688-03
37% Organically Bound Iodine
Diatrizoate Meglumine and Diatrizoate Sodium Solution USP
For oral or rectal use only
Do Not Inject
Rx only
120 mL
Guardian

NDC 53041-688-03
12 x 120 mL Bottle Shipper/case label
Diatrizoate Meglumine and Diatrizoate Sodium Solution USP
-
INGREDIENTS AND APPEARANCE
DIATRIZOATE MEGLUMINE AND DIATRIZOATE SODIUM
diatrizoate meglumine and diatrizoate sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53041-690 Route of Administration ORAL, RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIATRIZOATE MEGLUMINE (UNII: 3X9MR4N98U) (DIATRIZOIC ACID - UNII:5UVC90J1LK) DIATRIZOATE MEGLUMINE 660 mg in 1 mL DIATRIZOATE SODIUM (UNII: V5403H8VG7) (DIATRIZOIC ACID - UNII:5UVC90J1LK) DIATRIZOATE SODIUM 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color Score Shape Size Flavor LEMON Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53041-690-09 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 12/18/2019 2 NDC:53041-690-03 120 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 12/18/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214201 12/18/2019 DIATRIZOATE MEGLUMINE AND DIATRIZOATE SODIUM
diatrizoate meglumine and diatrizoate sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53041-688 Route of Administration ORAL, RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIATRIZOATE MEGLUMINE (UNII: 3X9MR4N98U) (DIATRIZOIC ACID - UNII:5UVC90J1LK) DIATRIZOATE MEGLUMINE 660 mg in 1 mL DIATRIZOATE SODIUM (UNII: V5403H8VG7) (DIATRIZOIC ACID - UNII:5UVC90J1LK) DIATRIZOATE SODIUM 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color Score Shape Size Flavor LEMON Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53041-688-03 120 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/18/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214201 12/18/2019 Labeler - Guardian Drug Company (119210276) Establishment Name Address ID/FEI Business Operations Guardian Drug Company 119210276 MANUFACTURE(53041-688, 53041-690)