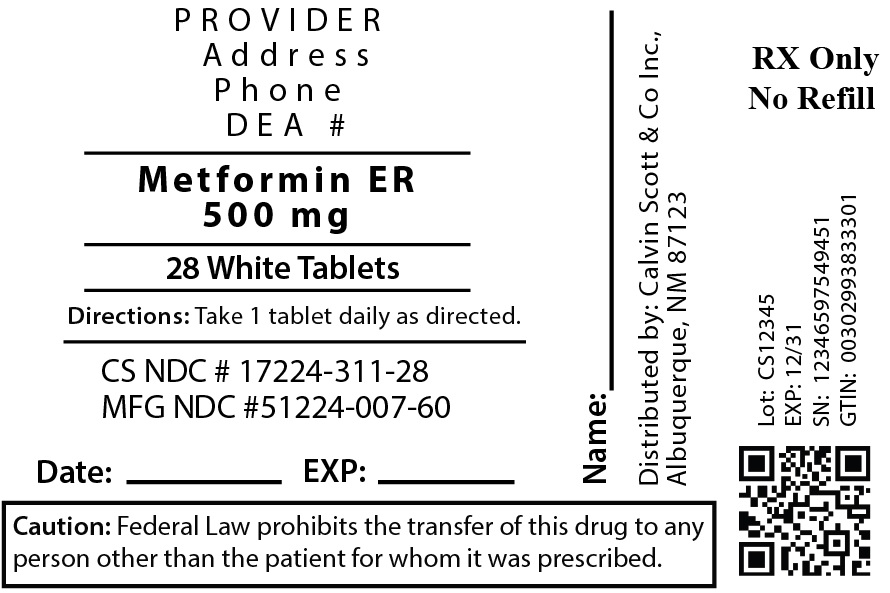
Label: METFORMIN HYDROCHLORIDE EXTENDED-RELEASE 500MG- metformin hydrochloride tablet, extended release
- NDC Code(s): 17224-311-28
- Packager: Calvin Scott & Co., Inc.
- This is a repackaged label.
- Source NDC Code(s): 51224-007
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated February 7, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
HOW TO USE THIS INFORMATION: This is a summary and does NOT have all possible information about this product. This information does not assure that this product is safe, effective, or appropriate for you. This information is not individual medical advice and does not substitute for the advice of your health care professional. Always ask your health care professional for complete information about this product and your specific health needs.
- SPL UNCLASSIFIED SECTION
-
WARNING:
Metformin can rarely cause a serious (sometimes fatal) condition called lactic acidosis. Stop taking metformin and get medical help right away if you develop any of the following symptoms of lactic acidosis: unusual tiredness, dizziness, severe drowsiness, chills, blue/cold skin, muscle pain, fast/difficult breathing, slow/irregular heartbeat, stomach pain with nausea, vomiting, or diarrhea. Lactic acidosis is more likely to occur in patients who have certain medical conditions, including kidney or liver disease, recent surgery, a serious infection, conditions that may cause a low level of oxygen in the blood or poor circulation (such as congestive heart failure, recent heart attack, recent stroke), heavy alcohol use, a severe loss of body fluids (dehydration), or X-ray or scanning procedures that require an injectable iodinated contrast drug. Tell your doctor immediately if any of these conditions occur or if you notice a big change in your overall health. You may need to stop taking this medication temporarily. The elderly are also at higher risk, especially those older than 80 years who have not had kidney tests. (See also Side Effects and Precautions sections.)
-
USES:
Metformin is used with a proper diet and exercise program and possibly with other medications to control high blood sugar. It is used in patients with type 2 diabetes (non-insulin-dependent diabetes). Controlling high blood sugar helps prevent kidney damage, blindness, nerve problems, loss of limbs, and sexual function problems. Proper control of diabetes may also lessen your risk of a heart attack or stroke. Metformin works by helping to restore your body's proper response to the insulin you naturally produce. It also decreases the amount of sugar that your liver makes and that your stomach/intestines absorb.
-
OTHER USES:
This section contains uses of this drug that are not listed in the approved professional labeling for the drug but that may be prescribed by your health care professional. Use this drug for a condition that is listed in this section only if it has been so prescribed by your health care professional. Metformin may be used with lifestyle changes such as diet and exercise to prevent diabetes in people who are at high risk for becoming diabetic. It is also used in women with a certain disease of the ovaries (polycystic ovarian syndrome). Metformin may make menstrual cycles more regular and increase fertility.
-
HOW TO USE:
Read the Patient Information Leaflet if available from your pharmacist before you start taking metformin and each time you get a refill. If you have any questions, consult your doctor or pharmacist. Take this medication by mouth as directed by your doctor, usually once daily with the evening meal. Drink plenty of fluids while taking this medication unless otherwise directed by your doctor. Do not crush or chew this medication. Doing so can release all of the drug at once, increasing the risk of side effects. Also, do not split the tablets unless they have a score line and your doctor or pharmacist tells you to do so. Swallow the whole or split tablet without crushing or chewing. The dosage is based on your medical condition, kidney function, and response to treatment. Your doctor may direct you to take a low dose of this medication at first, gradually increasing your dose to lower the chance of side effects such as upset stomach. Your doctor will adjust your dose based on your blood sugar levels to find the best dose for you. Follow your doctor's directions carefully. Take this medication regularly in order to get the most benefit from it. Remember to use it at the same time each day. If you are already taking another anti-diabetic drug (such as chlorpropamide), follow your doctor's directions carefully for stopping/continuing the old drug and starting metformin. Check your blood sugar regularly as directed by your doctor. Keep track of the results, and share them with your doctor. Tell your doctor if your blood sugar measurements are too high or too low. Your dosage/treatment may need to be changed.
-
SIDE EFFECTS:
Nausea, vomiting, stomach upset, diarrhea, weakness, or a metallic taste in the mouth may occur. If any of these effects persist or worsen, tell your doctor or pharmacist promptly. If stomach symptoms return later (after taking the same dose for several days or weeks), tell your doctor immediately. Stomach symptoms that occur after the first days of your treatment may be signs of lactic acidosis. An empty tablet shell may appear in your stool. This effect is harmless because your body has already absorbed the medication. Remember that your doctor has prescribed this medication because he or she has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects. Metformin does not usually cause low blood sugar (hypoglycemia). Low blood sugar may occur if this drug is prescribed with other anti-diabetic medications. Talk with your doctor or pharmacist about whether the dose of your other diabetic medication(s) needs to be lowered. Symptoms of low blood sugar include sudden sweating, shaking, fast heartbeat, hunger, blurred vision, dizziness, or tingling hands/feet. It is a good habit to carry glucose tablets or gel to treat low blood sugar. If you don't have these reliable forms of glucose, rapidly raise your blood sugar by eating a quick source of sugar such as table sugar, honey, or candy, or drink fruit juice or non-diet soda. Tell your doctor about the reaction immediately. Low blood sugar is more likely if you drink large amounts of alcohol, do unusually heavy exercise, or do not consume enough calories from food. To help prevent low blood sugar, eat meals on a regular schedule, and do not skip meals. Check with your doctor or pharmacist to find out what you should do if you miss a meal. Symptoms of high blood sugar (hyperglycemia) include thirst, increased urination, confusion, drowsiness, flushing, rapid breathing, and fruity breath odor. If these symptoms occur, tell your doctor immediately. Your doctor may need to adjust your diabetes medication(s). Stop taking this medication and tell your doctor right away if this very serious side effect occurs: lactic acidosis (see Warning section). A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any of the following symptoms of a serious allergic reaction: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing. This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist. In the US - Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
-
PRECAUTIONS:
See also Warning section. Before taking this medication, tell your doctor or pharmacist if you are allergic to metformin; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details. Before using this medication, tell your doctor or pharmacist your medical history, especially of: severe breathing problems (such as obstructive lung disease, severe asthma), metabolic acidosis (such as diabetic ketoacidosis), blood problems (such as anemia, vitamin B12 deficiency), kidney disease, liver disease. Before having surgery or any X-ray/scanning procedure using injectable iodinated contrast material, tell your doctor that you are taking this medication. You will need to temporarily stop this medication before the time of your surgery/procedure. Consult your doctor for further instructions. Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products). You may experience blurred vision, dizziness, or drowsiness due to extremely low or high blood sugar levels. Do not drive, use machinery, or do any activity that requires alertness or clear vision until you are sure you can perform such activities safely. Limit alcohol while using this medication because it can increase your risk of lactic acidosis and developing low blood sugar. High fever, "water pills" (diuretics such as hydrochlorothiazide), too much sweating, diarrhea, or vomiting may cause loss of too much body water (dehydration) and increase your risk of lactic acidosis. Stop taking this medication and tell your doctor right away if you have prolonged diarrhea or vomiting. Be sure to drink enough fluids to prevent dehydration unless your doctor directs you otherwise. It may be harder to control your blood sugar when your body is stressed (such as due to fever, infection, injury, or surgery). Consult your doctor because increased stress may require a change in your treatment plan, medications, or blood sugar testing. Older adults may be a greater risk for side effects such as low blood sugar or lactic acidosis. During pregnancy, this medication should be used only when clearly needed. Discuss the risks and benefits with your doctor. Your doctor may direct you to use insulin instead of this product during your pregnancy. Follow your doctor's instructions carefully. Metformin can cause changes in the menstrual cycle (promote ovulation) and increase the risk of becoming pregnant. Consult your doctor or pharmacist about the use of reliable birth control while using this medication. Metformin passes into breast milk in small amounts. Consult your doctor before breast-feeding.
-
DRUG INTERACTIONS:
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval. Many drugs can affect your blood sugar levels, making it more difficult to control your blood sugar. Before you start, stop, or change any medication, talk with your doctor or pharmacist about how the medication may affect your blood sugar. Check your blood sugar levels regularly as directed by your doctor. Tell your doctor about the results and of any symptoms of high or low blood sugar. (See also Side Effects section.) Your doctor may need to adjust your anti-diabetic medication, exercise program, or diet.
-
OVERDOSE:
If overdose is suspected, contact a poison control center or emergency room immediately. US residents can call the US National Poison Hotline at 1-800-222-1222. Canada residents can call a provincial poison control center. Overdose can cause lactic acidosis. Symptoms of overdose may include: severe drowsiness, severe nausea/vomiting/diarrhea, rapid breathing, slow/irregular heartbeat.
-
NOTES:
Do not share this medication with others. You should attend a diabetes education program to learn more about diabetes and all the important aspects of its treatment, including meals/diet, exercise, personal hygiene, medications, and getting regular eye/foot/medical exams. Keep all medical appointments. Laboratory and/or medical tests (such as liver/kidney function tests, blood glucose, hemoglobin A1c, complete blood counts) should be performed periodically to check for side effects and monitor your response to treatment. Check your blood sugar levels regularly as directed.
- MISSED DOSE:
-
STORAGE:
Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets. Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company for more details about how to safely discard your product.
- MEDICAL ALERT:
- SPL UNCLASSIFIED SECTION
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
METFORMIN HYDROCHLORIDE EXTENDED-RELEASE 500MG
metformin hydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17224-311(NDC:51224-007) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 500 mg Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape OVAL Size 19mm Flavor Imprint Code OE;584 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17224-311-28 28 in 1 BOTTLE; Type 0: Not a Combination Product 06/08/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078321 06/08/2017 Labeler - Calvin Scott & Co., Inc. (073404626)