Label: SOOTHE- pyridoxine hydrochloride, magnesium citrate, glycyrrhiza glabra, and ginger powder, for solution
- NHRIC Code(s): 69833-050-30
- Packager: Spring Hill Therapeutics LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated September 1, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
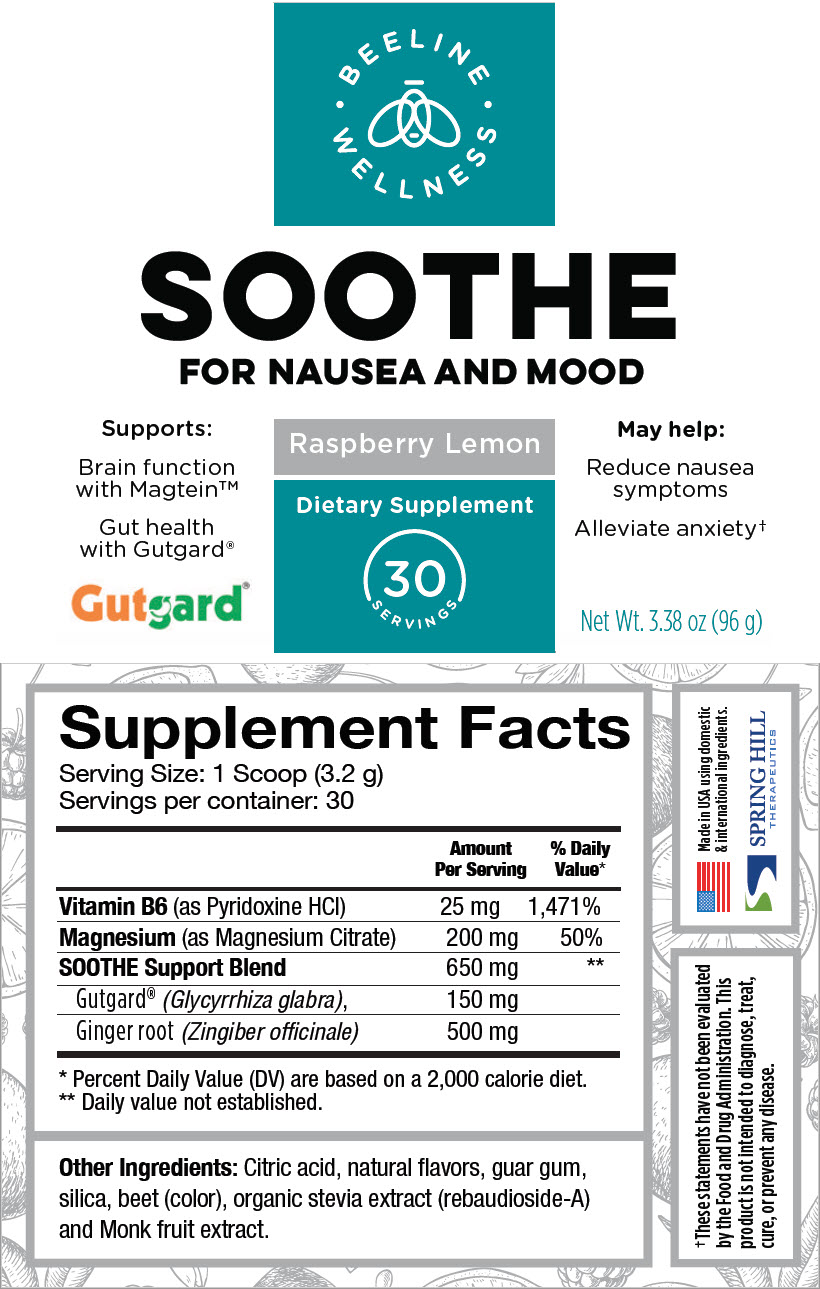
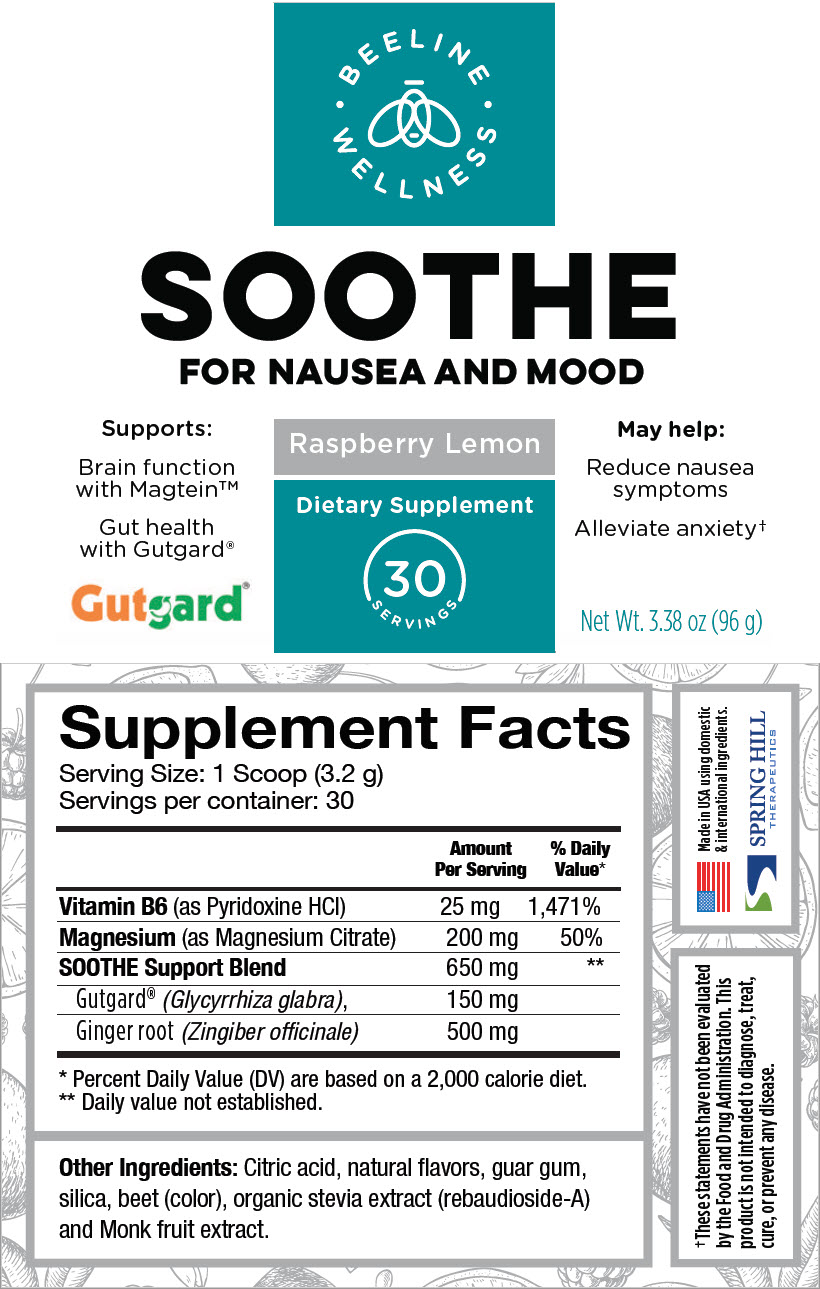
STATEMENT OF IDENTITY
Supplement Facts
Serving Size: 1 Scoop (3.2 g)
Servings per container: 30Amount Per Serving % Daily Value* Vitamin B6 (as Pyridoxine HCl) 25 mg 1,471% Magnesium (as Magnesium Citrate) 200 mg 50% SOOTHE Support Blend 650 mg † Gutgard® (Glycyrrhiza glabra), 150 mg Ginger root (Zingiber officinale) 500 mg Other Ingredients: Citric acid, natural flavors, guar gum, silica, beet (color), organic stevia extract (rebaudioside-A) and Monk fruit extract.
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 96 g Canister Label
-
INGREDIENTS AND APPEARANCE
SOOTHE
pyridoxine hydrochloride, magnesium citrate, glycyrrhiza glabra, and ginger powder, for solutionProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:69833-050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 25 mg in 3.2 g MAGNESIUM CITRATE (UNII: RHO26O1T9V) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CITRATE 200 mg in 3.2 g GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) (GLYCYRRHIZA GLABRA - UNII:2788Z9758H) GLYCYRRHIZA GLABRA 150 mg in 3.2 g GINGER (UNII: C5529G5JPQ) (GINGER - UNII:C5529G5JPQ) GINGER 500 mg in 3.2 g Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GUAR GUM (UNII: E89I1637KE) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) REBAUDIOSIDE A (UNII: B3FUD0528F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69833-050-30 96 g in 1 CANISTER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 07/01/2020 Labeler - Spring Hill Therapeutics LLC (079813250)