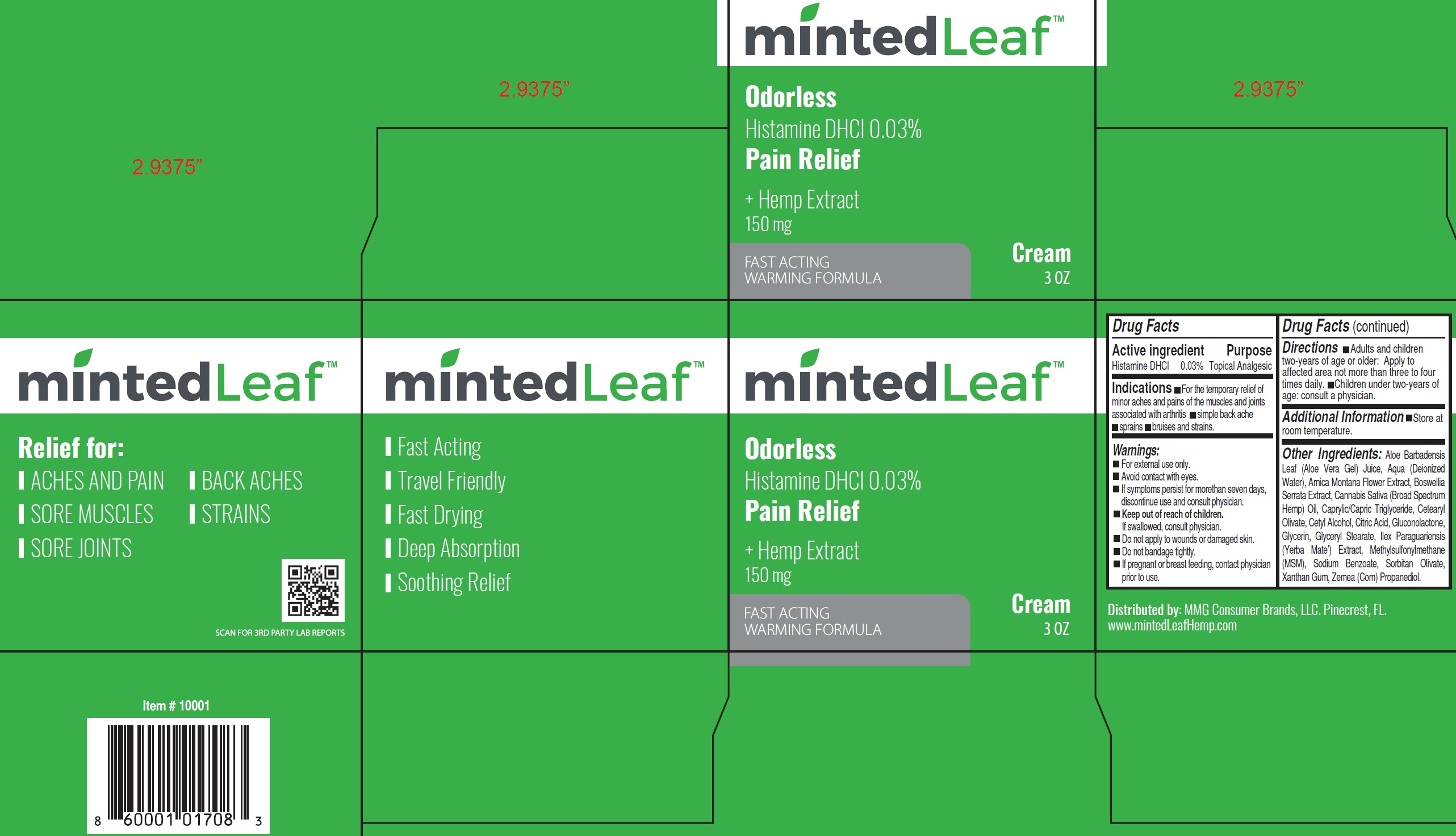
Label: MINTED LEAF ODORLESS PAIN RELIEF- histamine dihydrochloride cream
- NDC Code(s): 73102-118-03
- Packager: MMG Consumer Brands, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient:
- Indications:
- Warnings:
- Directions:
- Additional Information:
-
Other Ingredients:
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Arnica Montana Flower Extract, Boswellia Serrata Extract, Cannabis Sativa (Broad Spectrum Hemp) Oil, Caprylic/Capric Triglyceride, Cetearyl Olivate, Cetyl Alcohol, Citric Acid, Gluconolactone, Glycerin, Glyceryl Stearate, Ilex Paraguariensis (Yerba Mate’) Extract, Methylsulfonylmethane (MSM), Sodium Benzoate, Sorbitan Olivate, Xanthan Gum, Zemea (Corn) Propanediol.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
MINTED LEAF ODORLESS PAIN RELIEF
histamine dihydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73102-118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 0.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETEARYL OLIVATE (UNII: 58B69Q84JO) CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLUCONOLACTONE (UNII: WQ29KQ9POT) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITAN OLIVATE (UNII: MDL271E3GR) XANTHAN GUM (UNII: TTV12P4NEE) CORN (UNII: 0N8672707O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73102-118-03 1 in 1 BOX 05/01/2019 1 88 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/01/2019 Labeler - MMG Consumer Brands, LLC (117036455)