Label: CREAM- zinc oxide cream
- NDC Code(s): 78518-007-01
- Packager: MONAT GLOBAL CORP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
Directions
Directions
- Apply liberally 15 minutes before sun exposure
- Apply to sll skin exposed to the sun
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
-
Sun ProtectionMeasures
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10am-2pm
- wear a long-sleeved shirt, pants, hat, and sunglasses
-
INACTIVE INGREDIENT
Inactive Ingredients
Water/Eau, Dodecane, Coco-Caprylate, Tricaprylin, Brassica Napus Extract, Coconut Alkanes, Simmondsia Chinensis (Jojoba) Seed Oil, Stearalkonium Bentonite, Polyglceryl-3 Polyricinoleate, Oryza sativa (Rice) Bran Extract, Propanediol, Caprylic/Capric Triglyceride, Glyceryl Caprylate, Coco-caprylate/Caprate, Polyglyceryl-3 Diisostearate, Polyhydroxystearic Acid, Magnesium Sulfate, Dimethicone, Cocos Nucifera (Coconut) Oil, Citrus Limon (Lemon) Peel Oil, Citrus Aurantifolia (Lime) Oil, Citrus Aurantium Bergamia (Bergamot) Fruit Oil, Tremella Fuciformis Sporocarp Extract, Camellia Sinensis Leaf Extract, Linoleic Acid, Lecithin, Limnanthes Alba (Meadowfoam) Seed Oil, Crambe Abyssinica Seed Oil, Camellia Oleifera Seed Oil, Solanum Lycopersicum (Tomato) Seed Oil, Daucus Carota Sativa (Carrot) Seed Oil, Phytosteryl Canola Glycerides, Oleic Acid, Palmitic Acid, Stearic Acid, Triolein, Tocopherol, Adansonia Digitata Oil, Mauritia Flexuosa Fruit Oil, Gardenia Taitensis Flower Extract, Moringa Oleifera Seed Oil, Caryocar Brasiliense Fruit Oil, Helianthus Annuus (Sunflower) Seed Oil, Olea Europaea (Olive) Fruit Oil, Simmondsia Chinensis (Jojoba) Seed Extract, Triheptanoin, C9-12 Alkane, Dilinoleic Acid/Butanediol Copolymer, Castor Oil/IPDI Copolymer, Tocopherol Acetate, Ulva Lactuca Extract, Jojoba Esters, Polyglyceryl-6 Polyricinoleate, Silica, Triethyl Citrate, Glycerin, Betain, Glyceryl Undecylenate, Potassium Sorbate, Sodium Benzoate, Bentonite, May Contain: Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499).
- STORAGE AND HANDLING
- Primary Package(Tube)
- Outer Package(Carton)
-
INGREDIENTS AND APPEARANCE
CREAM
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78518-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 18.8 mg in 100 mg Inactive Ingredients Ingredient Name Strength CITRUS AURANTIIFOLIA FRUIT OIL (UNII: 7937R189CB) LINOLEIC ACID (UNII: 9KJL21T0QJ) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) LIMNANTHES ALBA WHOLE (UNII: DKY81513ER) CRAMBE HISPANICA SUBSP. ABYSSINICA SEED OIL (UNII: 0QW9S92J3K) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) WATER (UNII: 059QF0KO0R) DODECANE (UNII: 11A386X1QH) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) DIMETHICONE (UNII: 92RU3N3Y1O) COCOS NUCIFERA WHOLE (UNII: 245J88W96L) CITRUS LIMON SEED OIL (UNII: T78Z8273XO) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYDROLYZED JOJOBA ESTERS (POTASSIUM SALTS) (UNII: CH428W5O62) COCO-CAPRYLATE (UNII: 4828G836N6) DILINOLEIC ACID/BUTANEDIOL COPOLYMER (UNII: 1F2S8T535O) ULVA LACTUCA (UNII: PHR3P25W6Y) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 0.111 mg in 100 mg CITRUS AURANTIUM FRUIT OIL (UNII: 59JDQ5VT0T) TREMELLA FUCIFORMIS WHOLE (UNII: 4938BNS0GU) CAMELLIA SINENSIS WHOLE (UNII: C5M4585ZBZ) OLEIC ACID (UNII: 2UMI9U37CP) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL TRIOLEATE (UNII: O05EC62663) PHYTOSTERYL MACADAMIATE (UNII: 233VSF903M) TOCOPHEROL (UNII: R0ZB2556P8) CARYOCAR BRASILIENSE FRUIT OIL (UNII: WDI9AGC94F) TRIHEPTANOIN (UNII: 2P6O7CFW5K) SOLANUM LYCOPERSICUM (UNII: 0243Q4990L) DAUCUS CAROTA SUBSP. SATIVUS SEED (UNII: 9M6AAX381U) MAURITIA FLEXUOSA FRUIT OIL (UNII: 48H19MS04L) MORINGA OLEIFERA SEED OIL (UNII: REM6A5QMC0) HELIANTHUS ANNUUS WHOLE (UNII: 17S27ZT6KR) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) BRASSICA NAPUS WHOLE (UNII: NFP6814VZ4) COCONUT ALKANES (UNII: 1E5KJY107T) POLYGLYCERYL-5 POLYRICINOLEATE (UNII: FT3MR7CR64) PROPANEDIOL (UNII: 5965N8W85T) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SIMMONDSIA CHINENSIS SEED (UNII: D24K2Q1F6H) ORYZA SATIVA WHOLE (UNII: 84IVV0906Z) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAMELLIA OLEIFERA SEED (UNII: 59ED29FM2J) PALMITIC ACID (UNII: 2V16EO95H1) ADANSONIA DIGITATA SEED OIL (UNII: 77MKL7AR5I) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) CASTOR OIL (UNII: D5340Y2I9G) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL 1-UNDECYLENATE (UNII: B68LJT9544) SODIUM BENZOATE (UNII: OJ245FE5EU) BENTONITE (UNII: A3N5ZCN45C) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) BETAINE (UNII: 3SCV180C9W) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TRICAPRYLIN (UNII: 6P92858988) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78518-007-01 1 in 1 CARTON 09/12/2022 1 35 mg in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 09/12/2022 Labeler - MONAT GLOBAL CORP (027036949) Establishment Name Address ID/FEI Business Operations OXYGEN DEVELOPMENT, LLC 137098492 manufacture(78518-007)

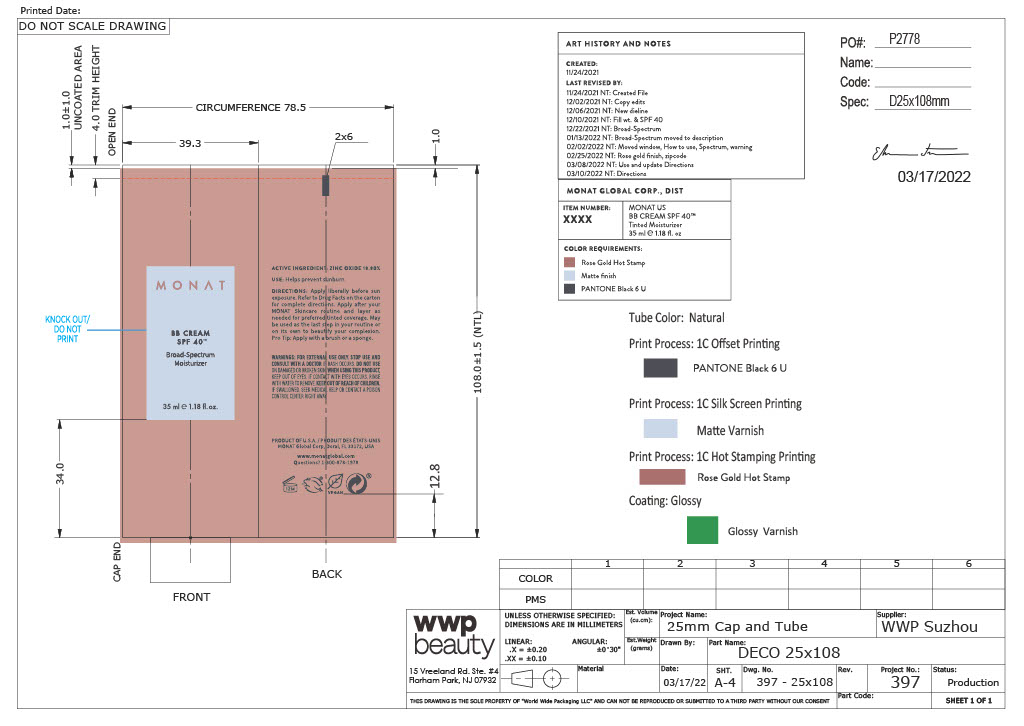
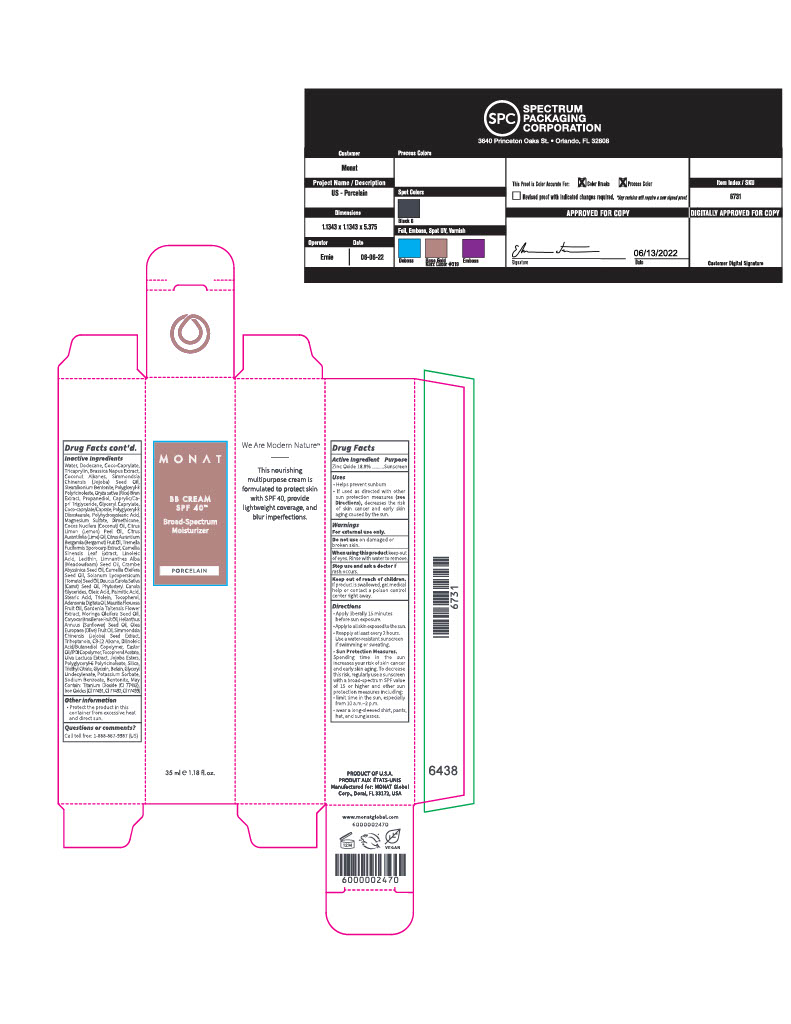
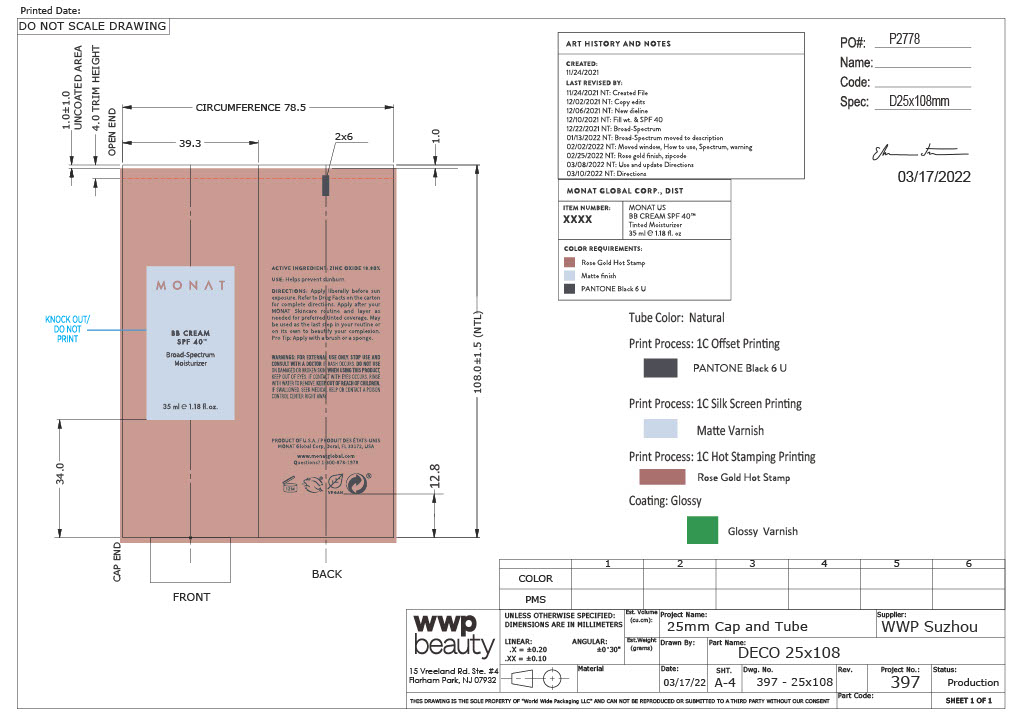
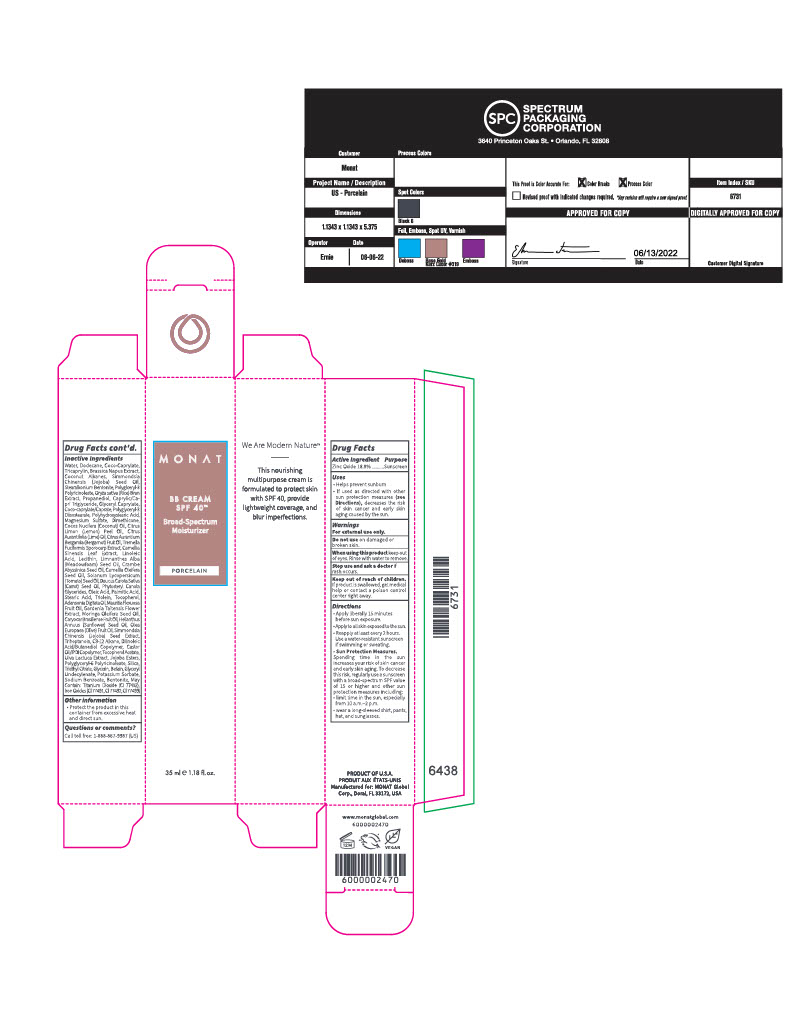 Carton- Outer Package
Carton- Outer Package