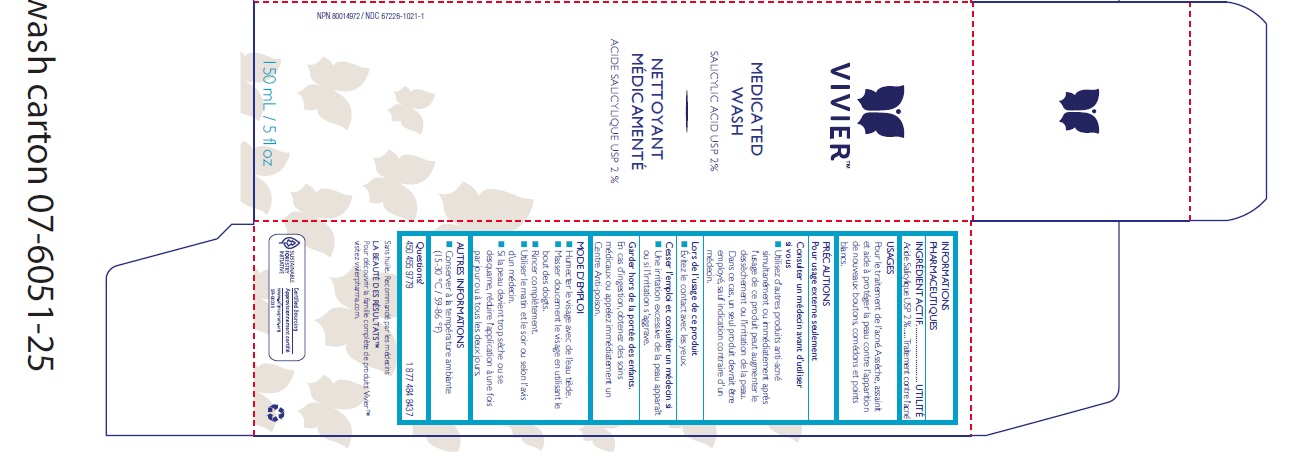
Label: MEDICATED WASH- salicylic acid liquid
- NDC Code(s): 67226-1021-1
- Packager: Vivier Pharma, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
WARNINGS
For exrernal use only
Ask a doctor before use if you are
- Using other topical acne medications at the
same time or immediately following use of
this product may increase dryness or
irritation of the skin. If this occurs, only one
medication should be used unless directed
by a doctor
When using this product
- Do not get into eyes.
Stop use and ask a doctor if
- Excessive skin irritation develops or increases.
- Using other topical acne medications at the
- Keep out of reach of children.
-
Directions
- Wet face using lukewarm water.
- Gently massage over the face using fingertips.
- Rinse completely.
- Use both morning and night or as directed by a physician.
- If dryness or peeling occurs, reduce application to once a day or every other day.
Other Information
- Store at room temperature
(15-30°C / 59-86°F).
- Inactive Ingredients
- Package Label
-
INGREDIENTS AND APPEARANCE
MEDICATED WASH
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67226-1021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) DISODIUM COCOYL GLUTAMATE (UNII: MBK0CP8F5A) DMDM HYDANTOIN (UNII: BYR0546TOW) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) HEXAMIDINE DIISETHIONATE (UNII: 023XA5Z50L) PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67226-1021-1 150 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 11/11/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/11/2017 Labeler - Vivier Pharma, Inc. (250996550) Establishment Name Address ID/FEI Business Operations Dermolab Pharma Ltd 245414743 manufacture(67226-1021)