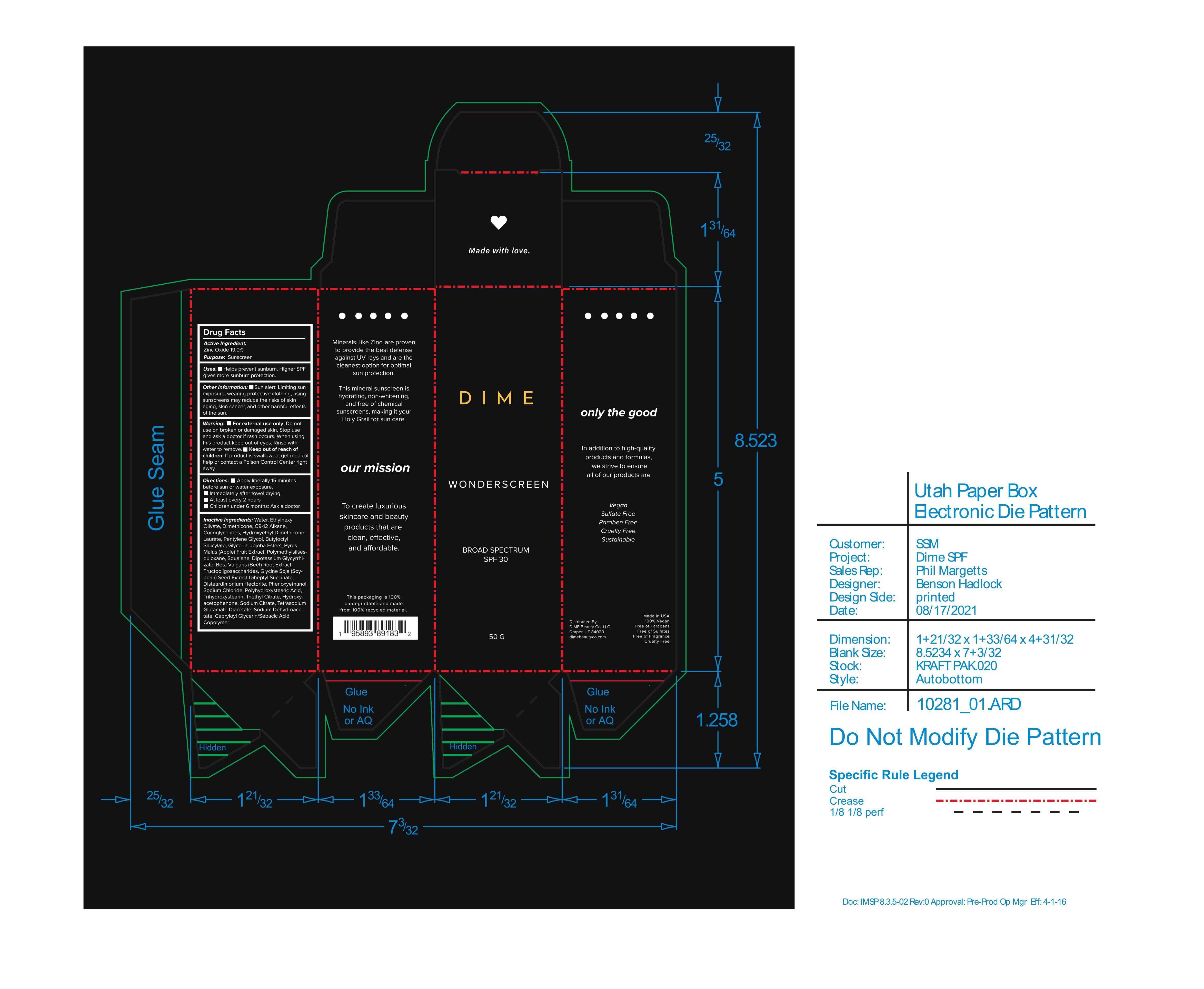
Label: WONDERSCREEN BROAD SPECTRUM SPF 30- zinc oxide cream
- NDC Code(s): 44717-062-01
- Packager: Wasatch Product Development, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients: Water, Ethylhexyl Olivate, Dimethicone, C9-12 Alkane, Cocoglycerides, Hydroxyethyl Dimethicone Laurate, Pentylene Glycol, Butyloctyl Salicylate, Glycerin, Jojoba Esters, Pyrus Malus (Apple) Fruit Extract, Polymethylsilsesquioxane, Squalane, Dipotassium Glycyrrhizate, Beta Vulgaris (Beet) Root Extract, Fructooligosaccharides, Glycine Soja (Soybean) Seed Extract Diheptyl Succinate, Disteardimonium Hectorite, Phenoxyethanol, Sodium Chloride, Polyhydroxystearic Acid, Trihydroxystearin, Triethyl Citrate, Hydroxyacetophenone, Sodium Citrate, Tetrasodium Glutamate Diacetate, Sodium Dehydroacetate, Capryloyl Glycerin/Sebacic Acid Copolymer
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WONDERSCREEN BROAD SPECTRUM SPF 30
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44717-062 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 19 g in 100 g Inactive Ingredients Ingredient Name Strength COCO-GLYCERIDES (UNII: ISE9I7DNUG) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) PENTYLENE GLYCOL (UNII: 50C1307PZG) DIMETHICONE 100 (UNII: RO266O364U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) SQUALANE (UNII: GW89575KF9) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) BEET (UNII: N487KM8COK) WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) GLYCERIN (UNII: PDC6A3C0OX) APPLE (UNII: B423VGH5S9) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PHENOXYETHANOL (UNII: HIE492ZZ3T) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44717-062-01 50 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 02/25/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 02/25/2022 Labeler - Wasatch Product Development, LLC. (962452533)