Label: DR TALBOTS INFANT COUGH RELIEF- antimonium tartaricum 12x,drosera rotundifolia 12x,ipecacuanha 12x,phosphorus 12x,rumex crispus 12x,spongia tosta 12x,sticta pulmonaria 12x,kali sulphuricum 6x liquid
- NDC Code(s): 70797-319-01
- Packager: Talbot’s Pharmaceuticals Family Products, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated September 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- ASK A DOCTOR
- PREGNANCY
- KEEP OUT OF REACH OF CHILDREN SECTION
- INACTIVE INGREDIENTS
-
DOSAGE
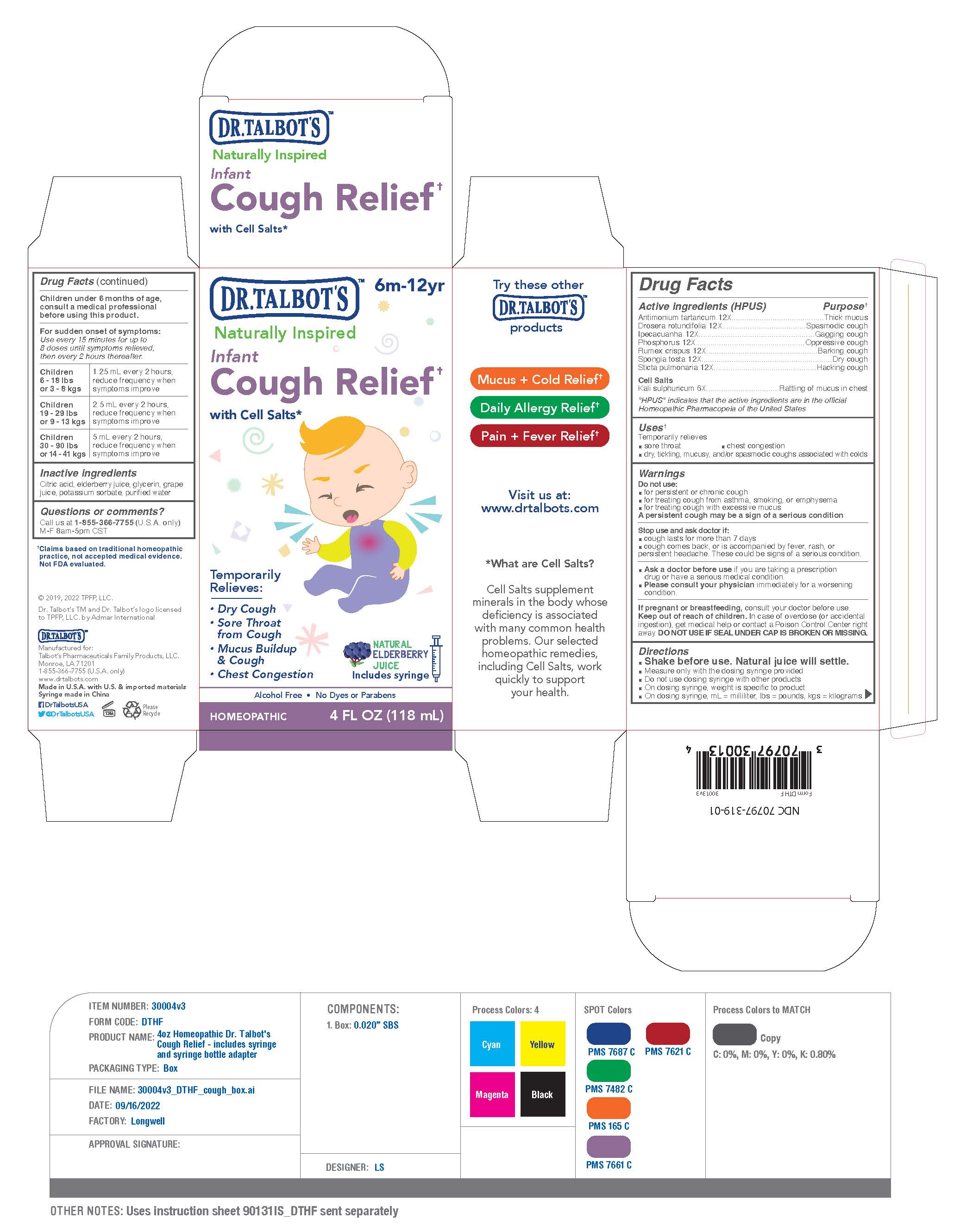 Children under 6 months of age, consult a medical professional before using this product.(SEE ATTCHED PRODUCT IMAGE)
Children under 6 months of age, consult a medical professional before using this product.(SEE ATTCHED PRODUCT IMAGE)
For sudden onset of symptoms: Use every 15 minutes for up to 8 doses until symptoms relieved, then every 2 hours thereafter.
Children 6 - 18 lbs or 3 - 8 kgs
1.25 ml every 2 hours, reduce frequency when symptoms improve
Children 19 - 29 lbs or 9 - 13 kgs
2.5 ml every 2 hours, reduce frequency when symptoms improve
Children 30 - 90 lbs or 14 - 41 kgs
5 ml every 2 hours, reduce frequency when symptoms improve
- QUESTION
-
INSTRUCTIONS
 For sudden onset of symptoms: (SEE ATTCHED PRODUCT LABEL IMAGE)
For sudden onset of symptoms: (SEE ATTCHED PRODUCT LABEL IMAGE)
Use every 15 minutes for up to
8 doses until symptoms relieved,
then every 2 hours thereafter.Children
6 - 18 lbs
or 3 - 8 kgs1.25 mL every 2 hours,
reduce frequency when
symptoms improveChildren
19 - 29 lbs
or 9 - 13 kgs2.5 mL every 2 hours,
reduce frequency when
symptoms improveChildren
30 - 90 lbs
or 14 - 41 kgs5 mL every 2 hours,
reduce frequency when
symptoms improve - Warnings
- Purpose
- Usage
- PRINCIPAL DISPLAY
-
INGREDIENTS AND APPEARANCE
DR TALBOTS INFANT COUGH RELIEF
antimonium tartaricum 12x,drosera rotundifolia 12x,ipecacuanha 12x,phosphorus 12x,rumex crispus 12x,spongia tosta 12x,sticta pulmonaria 12x,kali sulphuricum 6x liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70797-319 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 12 [hp_X] in 118 mL PULMONARIA OFFICINALIS WHOLE (UNII: 483B0A2Y00) (PULMONARIA OFFICINALIS WHOLE - UNII:483B0A2Y00) PULMONARIA OFFICINALIS WHOLE 12 [hp_X] in 118 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 12 [hp_X] in 118 mL ANTIMONY TARTRATE ION (UNII: CQN5KB2U1M) (ANTIMONY TARTRATE ION - UNII:CQN5KB2U1M) ANTIMONY TARTRATE ION 12 [hp_X] in 118 mL SPONGIA OFFICINALIS SKELETON, ROASTED (UNII: 1PIP394IID) (SPONGIA OFFICINALIS SKELETON, ROASTED - UNII:1PIP394IID) SPONGIA OFFICINALIS SKELETON, ROASTED 12 [hp_X] in 118 mL RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) (RUMEX CRISPUS ROOT - UNII:9N1RM2S62C) RUMEX CRISPUS ROOT 12 [hp_X] in 118 mL DROSERA ROTUNDIFOLIA FLOWERING TOP (UNII: 75O014T1HG) (DROSERA ROTUNDIFOLIA FLOWERING TOP - UNII:75O014T1HG) DROSERA ROTUNDIFOLIA FLOWERING TOP 12 [hp_X] in 118 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) EUROPEAN ELDERBERRY JUICE (UNII: Z4IFJ0AK1E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CONCORD GRAPE JUICE (UNII: F7039Q79LP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70797-319-01 1 in 1 CARTON 09/12/2022 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/12/2022 Labeler - Talbot’s Pharmaceuticals Family Products, LLC. (078855555)

