Label: CHILDRENS ALLERGY- diphenhydramine hcl solution
- NDC Code(s): 69676-0074-9
- Packager: Genexa Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to enlargement of the prostate gland
-
DOSAGE & ADMINISTRATION
Directions
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 doses in 24 hours
- use only with enclosed dose cup. Do not use with any other dosing device.
adults and children 12 years of age and over 10 - 20 mL children 6 to under 12 years 5 - 10 mL children 2 to under 6 years do not use unless directed by a doctor children under 2 years do not use - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
*This product is not manufactured or distributed by
Johnson & Johnson Corp., owner of the registered
trademark Children's Benadryl® Allergy Liquid Cherry.
Bottle is safety sealed with a perforated band around bottle.
DO NOT use if bottle seal is disturbed or missing.
Patent Pending | Distributed by: Genexa Inc.
Atlanta, GA 30318 | genexa.com
- SPL UNCLASSIFIED SECTION
-
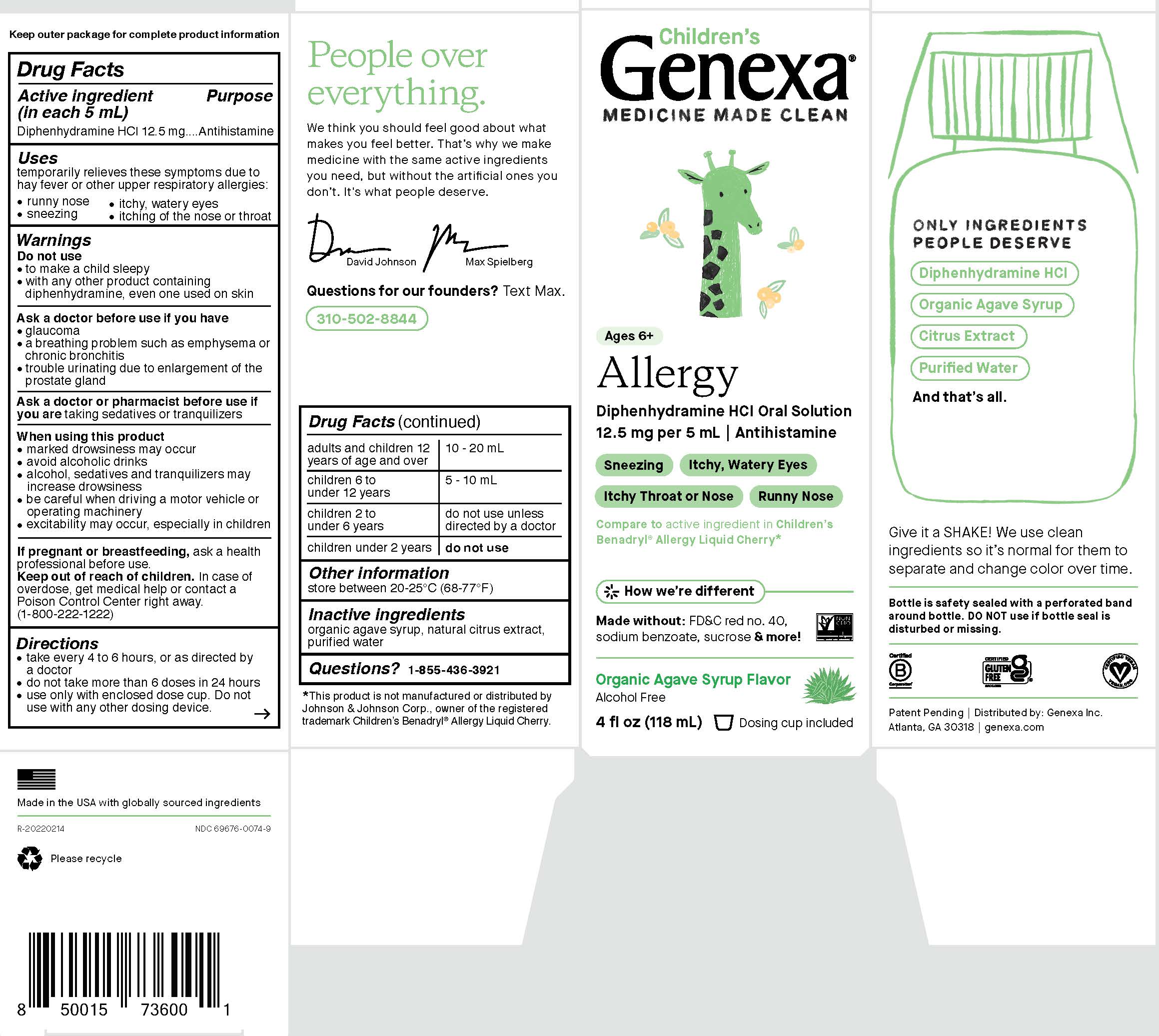
Children's Allergy
Children's
Genexa®
MEDICINE MADE CLEAN
Ages 6+
Allergy
Diphenhydramine HCl Oral Solution
12.5 mg per 5 mL | Antihistamine
Sneezing
Itchy, Watery Eyes
Itchy Throat or Nose
Runny Nose
Compareto active ingredient in Children’s
Benadryl® Allergy Liquid Cherry*How we're different
Made without:
FD&C red no. 40,
sodium benzoate, sucrose & more!
Organic Agave Syrup Flavor
Alcohol Free
4 FL OZ (118 mL) Dosing cup included
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLERGY
diphenhydramine hcl solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69676-0074 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength AGAVE TEQUILANA JUICE (UNII: GVG8G0207O) WATER (UNII: 059QF0KO0R) CITRUS FRUIT (UNII: XDK00Z8012) Product Characteristics Color brown (Golden) Score Shape Size Flavor HONEY (Organic Agave Syrup Flavor) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69676-0074-9 1 in 1 CARTON 04/26/2022 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 04/26/2022 Labeler - Genexa Inc. (079751024)

