Label: 5 PERCENT BENZOYL PEROXIDE CLEANSER- benzoyl peroxide gel
- NDC Code(s): 81136-023-01
- Packager: Brand Evangelists for Beauty Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
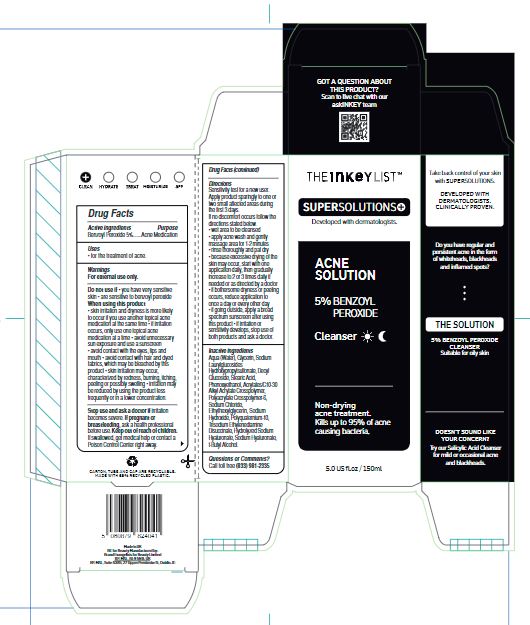
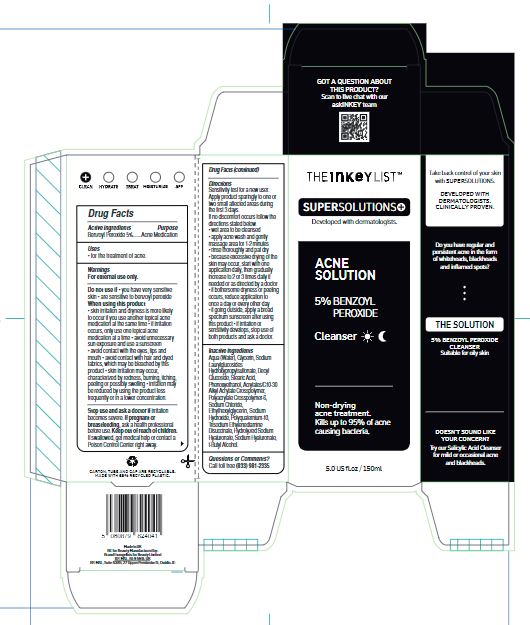
- Drug Facts
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only.
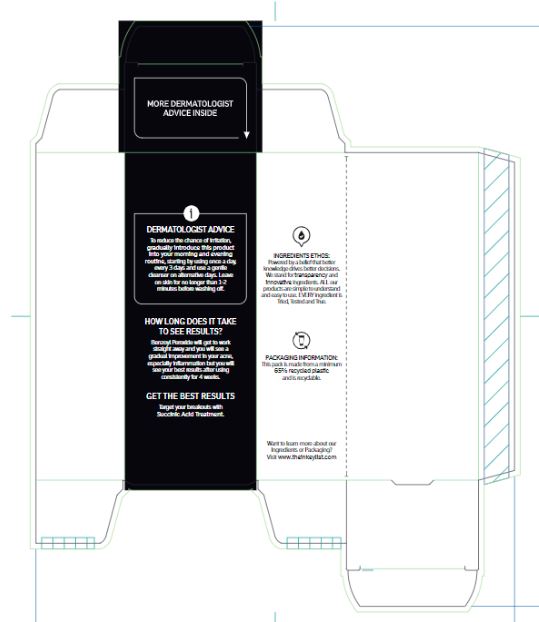
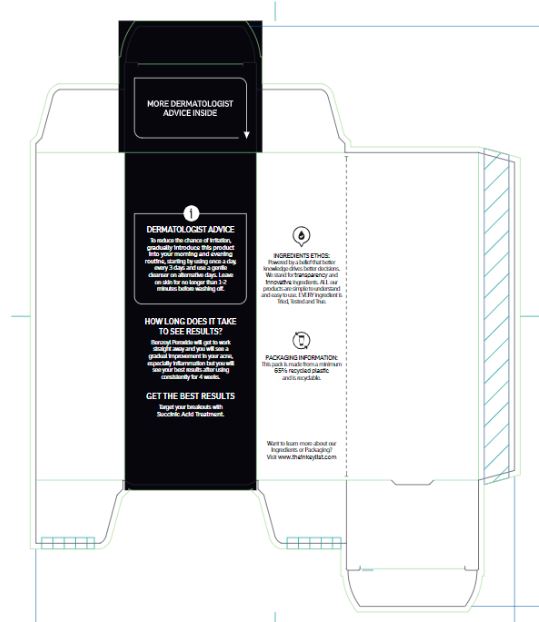
When using this product
• skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time • if irritation occurs, only use one topical acne medication at a time • avoid unnecessary sun exposure and use a sunscreen • avoid contact with the eyes, lips and mouth • avoid contact with hair and dyed fabrics, which may be bleached by this product • skin irritation may occur, characterized by redness, burning, itching, peeling or possibly swelling • irritation may be reduced by using the product less frequently or in a lower concentration.
-
Directions
Sensitivity test for a new user. Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs follow the directions stated below.• wet area to be cleansed • apply acne wash and gently massage area for 1-2 minutes • rinse thoroughly and pat dry • because excessive drying of the skin may occur, start with one application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor • if bothersome dryness or peeling occurs, reduce application to once a day or every other day • if going outside, apply a broad spectrum sunscreen after using this product • if irritation or sensitivity develops, stop use of both products and ask a doctor.
-
Inactive ingredients
Aqua (Water), Glycerin, Sodium Laurylglucosides Hydroxypropylsulfonate, Decyl Glucoside, Stearic Acid, Phenoxyethanol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polyacrylate Crosspolymer-6, Sodium Chloride, Ethylhexylglycerin, SodiumHydroxide, Polyquaternium-10, Trisodium Ethylenediamine Disuccinate, Hydrolyzed Sodium Hyaluronate, Sodium Hyaluronate, t-Butyl Alcohol.
- Questions or Comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
5 PERCENT BENZOYL PEROXIDE CLEANSER
benzoyl peroxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81136-023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM LAUROYL HYDROXYPROPYL SULFONATE (UNII: IQ398K5X8M) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) STEARIC ACID (UNII: 4ELV7Z65AP) PHENOXYETHANOL (UNII: HIE492ZZ3T) CARBOMER INTERPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 132584PQMO) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) SODIUM CHLORIDE (UNII: 451W47IQ8X) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM HYDROXIDE (UNII: 55X04QC32I) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81136-023-01 1 in 1 CARTON 08/24/2022 1 150 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/24/2022 Labeler - Brand Evangelists for Beauty Limited (222990724) Registrant - Orean Personal Care Ltd. (211403363) Establishment Name Address ID/FEI Business Operations Orean Personal Care Ltd. 211403363 manufacture(81136-023)