Label: BULL FROG- avobenzone, octocrylene, homosalate, octyl methoxycinnamate, octisalate spray
- NDC Code(s): 58443-0575-4
- Packager: Prime Enterprises Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
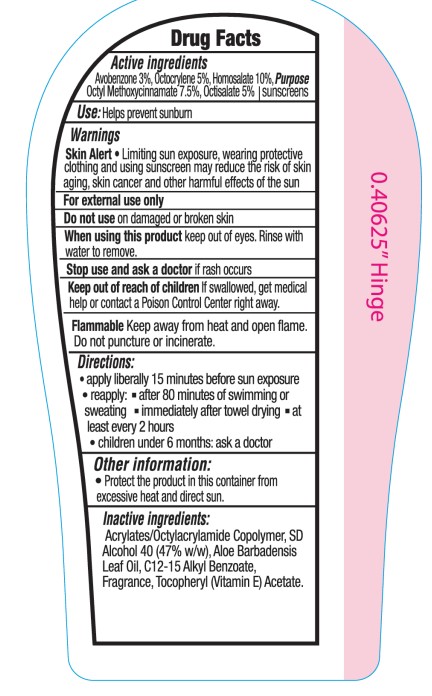
- Active ingredients
- Purpose
- Use:
- Warnings
- Directions
- Other information
- Inactive ingredients:
- Questions or comments?
- BullFrog SPF 50 Mosquito Coast Sunscreen + Insect Repellent
-
INGREDIENTS AND APPEARANCE
BULL FROG
avobenzone, octocrylene, homosalate, octyl methoxycinnamate, octisalate sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58443-0575 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 44.25 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 88.5 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 66.4 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 26.55 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 44.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALCOHOL (UNII: 3K9958V90M) ETHYL BUTYLACETYLAMINOPROPIONATE (UNII: 65GQA237EH) ACRYLATES/OCTYLACRYLAMIDE COPOLYMER (40000 MW) (UNII: 7LL6SY9YFV) Product Characteristics Color yellow (Very Light Yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58443-0575-4 138 mL in 1 TUBE; Type 0: Not a Combination Product 12/03/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/03/2021 Labeler - Prime Enterprises Inc. (101946028) Registrant - Prime Enterprises Inc. (101946028) Establishment Name Address ID/FEI Business Operations Prime Enterprises Inc. 101946028 pack(58443-0575) , manufacture(58443-0575) , label(58443-0575) , analysis(58443-0575)