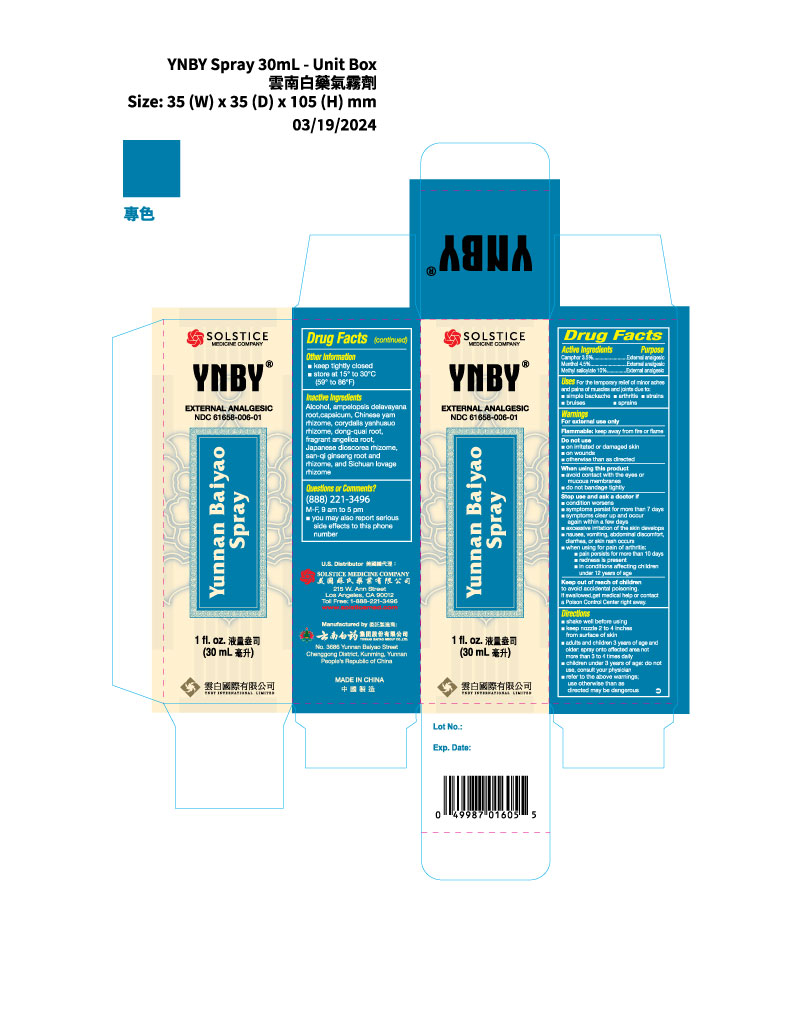
Label: YUNNAN BAIYAO- camphor, menthol, and methyl salicylate spray
- NDC Code(s): 61658-006-01, 61658-006-02, 61658-006-03
- Packager: YUNNAN BAIYAO GROUP CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
condition worsens
symptoms persist for more than 7 days
symptoms clear up and occur again within a few days
excessive irritation of the skin develops
nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
when using for pain of arthritis:
pain persists for more than 10 days
redness is present
in conditions affecting children under 12 years of age - KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
shake well before using
adults and children 3 years of age and older: remove the attached film from the patch. Apply to affected area not more than 3 to 4 times daily
children under 3 years of age: do not use, consult your physicianrefer to the above warnings; use otherwise than as directed may be dangerous - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
YUNNAN BAIYAO
camphor, menthol, and methyl salicylate sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61658-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 3.5 g in 100 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 4.5 g in 100 mL METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength AMPELOPSIS DELAVAYANA ROOT (UNII: N93790DMR5) CAPSICUM (UNII: 00UK7646FG) CHINESE YAM (UNII: 29CIF30B1Z) CORYDALIS YANHUSUO WHOLE (UNII: DX4V4VDT5J) ANGELICA SINENSIS ROOT (UNII: B66F4574UG) ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) DIOSCOREA JAPONICA WHOLE (UNII: 1I0396UIN0) PANAX NOTOGINSENG WHOLE (UNII: E7XOU43ESD) LIGUSTICUM SINENSE SUBSP. CHUANXIONG WHOLE (UNII: CAX256379F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61658-006-01 1 in 1 BOX 03/19/2024 1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 2 NDC:61658-006-02 1 in 1 BOX 03/19/2024 2 85 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 3 NDC:61658-006-03 1 in 1 BOX 05/10/2024 3 50 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug part348 03/19/2024 Labeler - YUNNAN BAIYAO GROUP CO., LTD. (654223122) Establishment Name Address ID/FEI Business Operations YUNNAN BAIYAO GROUP CO., LTD. 654223122 manufacture(61658-006)