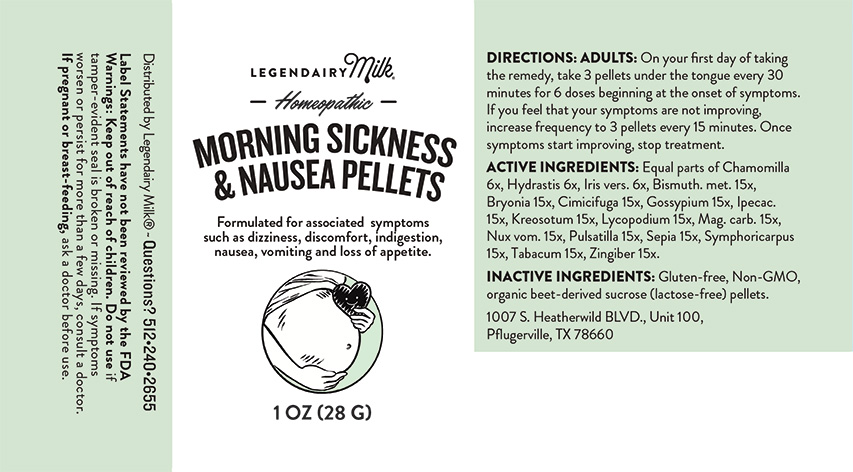
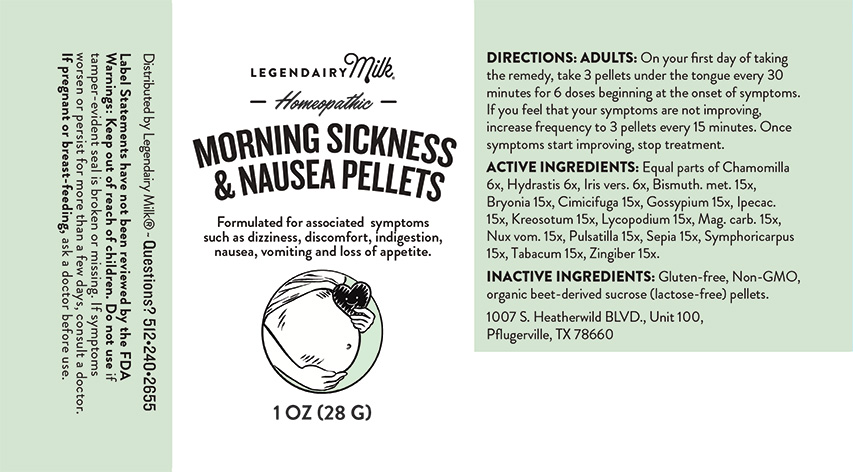
Label: MORNING SICKNESS AND NAUSEA PELLETS- chamomilla, hydrastis canadensis, iris versicolor, bismuthum metallicum, bryonia, cimicifuga racemosa, gossypium herbaceum, ipecacuanha, kreosotum, lycopodium clavatum, magnesia carbonica, nux vomica, pulsatilla, sepia, symphoricarpus racemosus, tabacum, zingiber officinale. pellet
- NDC Code(s): 82952-0001-1
- Packager: Legendairy Milk
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated September 8, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE SECTION
-
DOSAGE & ADMINISTRATION SECTION
Directions: Adults: On your first day of taking the remedy, take 3 pellets under the tongue every 30 minutes for 6 doses beginning at the onset of symptoms. If you feel that your symptoms are not improving, increase frequency to 3 pellets every 15 minutes. Once symptoms start improving, stop treatment.
- OTC - ACTIVE INGREDIENT SECTION
- OTC - PURPOSE SECTION
- INACTIVE INGREDIENT SECTION
- QUESTIONS SECTION
- WARNINGS SECTION
- OTC - PREGNANCY OR BREAST FEEDING SECTION
- OTC - KEEP OUT OF REACH OF CHILDREN SECTION
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
MORNING SICKNESS AND NAUSEA PELLETS
chamomilla, hydrastis canadensis, iris versicolor, bismuthum metallicum, bryonia, cimicifuga racemosa, gossypium herbaceum, ipecacuanha, kreosotum, lycopodium clavatum, magnesia carbonica, nux vomica, pulsatilla, sepia, symphoricarpus racemosus, tabacum, zingiber officinale. pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82952-0001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IRIS VERSICOLOR ROOT (UNII: X43D4L3DQC) (IRIS VERSICOLOR ROOT - UNII:X43D4L3DQC) IRIS VERSICOLOR ROOT 6 [hp_X] in 1 g BISMUTH (UNII: U015TT5I8H) (BISMUTH - UNII:U015TT5I8H) BISMUTH 15 [hp_X] in 1 g BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 15 [hp_X] in 1 g BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 15 [hp_X] in 1 g GOSSYPIUM HERBACEUM ROOT BARK (UNII: VQN1631P4C) (GOSSYPIUM HERBACEUM ROOT BARK - UNII:VQN1631P4C) GOSSYPIUM HERBACEUM ROOT BARK 15 [hp_X] in 1 g IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 15 [hp_X] in 1 g WOOD CREOSOTE (UNII: 3JYG22FD73) (WOOD CREOSOTE - UNII:3JYG22FD73) WOOD CREOSOTE 15 [hp_X] in 1 g LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 15 [hp_X] in 1 g MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 15 [hp_X] in 1 g STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 15 [hp_X] in 1 g MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 6 [hp_X] in 1 g SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 15 [hp_X] in 1 g SYMPHORICARPOS ALBUS FRUIT (UNII: 873JZU3ASZ) (SYMPHORICARPOS ALBUS FRUIT - UNII:873JZU3ASZ) SYMPHORICARPOS ALBUS FRUIT 15 [hp_X] in 1 g TOBACCO LEAF (UNII: 6YR2608RSU) (TOBACCO LEAF - UNII:6YR2608RSU) TOBACCO LEAF 15 [hp_X] in 1 g GINGER (UNII: C5529G5JPQ) (GINGER - UNII:C5529G5JPQ) GINGER 15 [hp_X] in 1 g GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_X] in 1 g ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 15 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82952-0001-1 28 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 09/08/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/08/2022 Labeler - Legendairy Milk (006228879) Registrant - Newton Laboratories, Inc. (788793610) Establishment Name Address ID/FEI Business Operations Newton Laboratories, Inc. 788793610 manufacture(82952-0001)