Label: UREA 39% CREAM cream
- NDC Code(s): 44523-801-08
- Packager: BioComp Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- GENERAL:
-
DESCRIPTION
This product is a keratolytic emollient which is a gentle, yet potent, tissue softener for skin.
Each gram contains 390 mg of urea in a vehicle consisting of: carbomer, cetyl alcohol, dimethyl isosorbide, glyceryl stearate, mineral oil, petrolatum, propylene glycol, sodium hydroxide, water, and xanthan gum.
Urea is a diamide of carbonic acid with the following chemical structure:
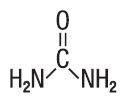
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS:
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
-
PREGNANCY
Category C.Animal reproduction studies have not been conducted with this product. It is also not known whether this product can affect reproduction capacity or cause fetal harm when administered to a pregnant woman. This product should be used by a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus.
- NURSING MOTHERS
- ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- NOTICE:
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UREA 39% CREAM
urea 39% cream creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:44523-801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UREA (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) UREA 390 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) XANTHAN GUM (UNII: TTV12P4NEE) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) CETYL ALCOHOL (UNII: 936JST6JCN) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44523-801-08 227 g in 1 BOTTLE; Type 0: Not a Combination Product 09/23/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/23/2022 Labeler - BioComp Pharma, Inc. (829249718) Establishment Name Address ID/FEI Business Operations Mission Pharmacal Company 927726893 manufacture(44523-801)


