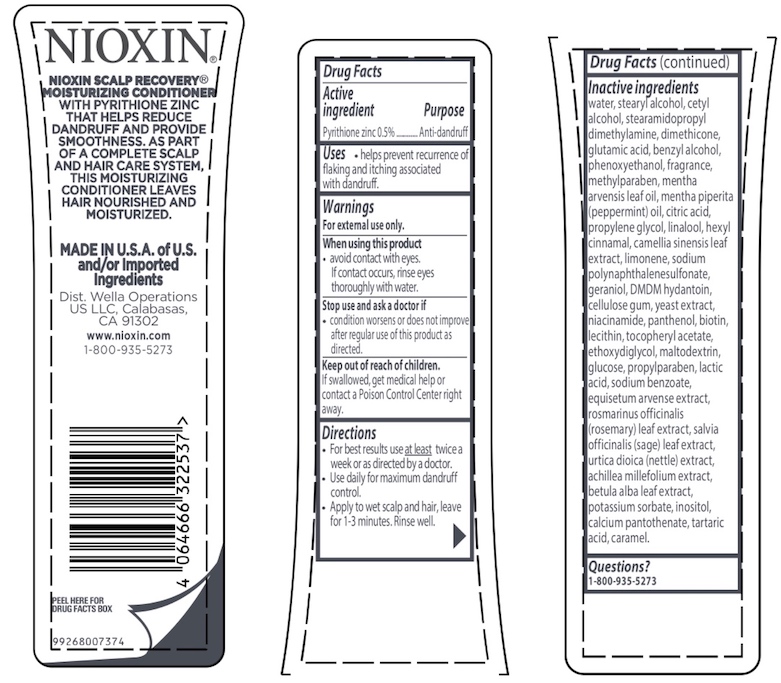
Label: NIOXIN SCALP RECOVERY MOISTURIZING CONDITIONER- pyrithione zinc lotion
- NDC Code(s): 82157-004-20
- Packager: Wella Operations US LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients water, stearyl alcohol, cetyl alcohol, stearamidopropyl dimethylamine, dimethicone, glutamic acid, benzyl alcohol, phenoxyethanol, fragrance, methylparaben, mentha arvensis leaf oil, mentha piperita (peppermint) oil, citric acid, propylene glycol, linalool, hexyl cinnamal, camellia sinensis leaf extract, limonene, sodium polynaphthalenesulfonate, geraniol, DMDM hydantoin, cellulose gum, yeast extract, niacinamide, panthenol, biotin, lecithin, tocopheryl acetate, ethoxydiglycol, maltodextrin, glucose, propylparaben, lactic acid, sodium benzoate, equisetum arvense extract, rosmarinus officinalis (rosemary) leaf extract, salvia officinalis (sage) leaf extract, urtica dioica (nettle) extract, achillea millefolium extract, betula alba leaf extract, potassium sorbate, inositol, calcium pantothenate, tartaric acid, caramel.
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
NIOXIN SCALP RECOVERY® MOISTURIZING CONDITIONER
WITH PYRITHIONE ZINC THAT HELPS REDUCE DANDRUFF AND PROVIDE SMOOTHNESS. AS PART OF A COMPLETE SCALP AND HAIR CARE SYSTEM, THIS MOISTURIZING CONDITIONER LEAVES HAIR NOURISHED AND MOISTURIZED.
MADE IN U.S.A. of U.S. and/or imported ingredients
Dist. Wella Operations US LLC, Calabasas, CA 91302
www.nioxin.com
1-800-935-5273
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NIOXIN SCALP RECOVERY MOISTURIZING CONDITIONER
pyrithione zinc lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82157-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength FORMALDEHYDE/SODIUM NAPHTHALENESULFONATE COPOLYMER (3000 MW) (UNII: 90D834OZUI) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) CETYL ALCOHOL (UNII: 936JST6JCN) STEARAMIDOPROPYL DIMETHYLAMINE (UNII: K7VEI00UFR) DIMETHICONE (UNII: 92RU3N3Y1O) GLUTAMIC ACID (UNII: 3KX376GY7L) BENZYL ALCOHOL (UNII: LKG8494WBH) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PEPPERMINT OIL (UNII: AV092KU4JH) MENTHA ARVENSIS LEAF OIL (UNII: 1AEY1M553N) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) BETULA PUBESCENS LEAF (UNII: 84SOH0O3OO) INOSITOL (UNII: 4L6452S749) TARTARIC ACID (UNII: W4888I119H) NIACINAMIDE (UNII: 25X51I8RD4) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIMONENE, (+/-)- (UNII: 9MC3I34447) ROSEMARY (UNII: IJ67X351P9) DMDM HYDANTOIN (UNII: BYR0546TOW) LACTIC ACID (UNII: 33X04XA5AT) SODIUM BENZOATE (UNII: OJ245FE5EU) CARAMEL (UNII: T9D99G2B1R) LINALOOL, (+/-)- (UNII: D81QY6I88E) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) URTICA DIOICA LEAF (UNII: X6M0DRN46Q) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) GERANIOL (UNII: L837108USY) PANTHENOL (UNII: WV9CM0O67Z) BIOTIN (UNII: 6SO6U10H04) MALTODEXTRIN (UNII: 7CVR7L4A2D) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) SAGE (UNII: 065C5D077J) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82157-004-20 200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M032 12/01/2022 Labeler - Wella Operations US LLC (117781338)