Label: MEDERMA FOR KIDS- allantoin gel
- NDC Code(s): 73302-202-20
- Packager: HRA PHARMA AMERICA, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments?
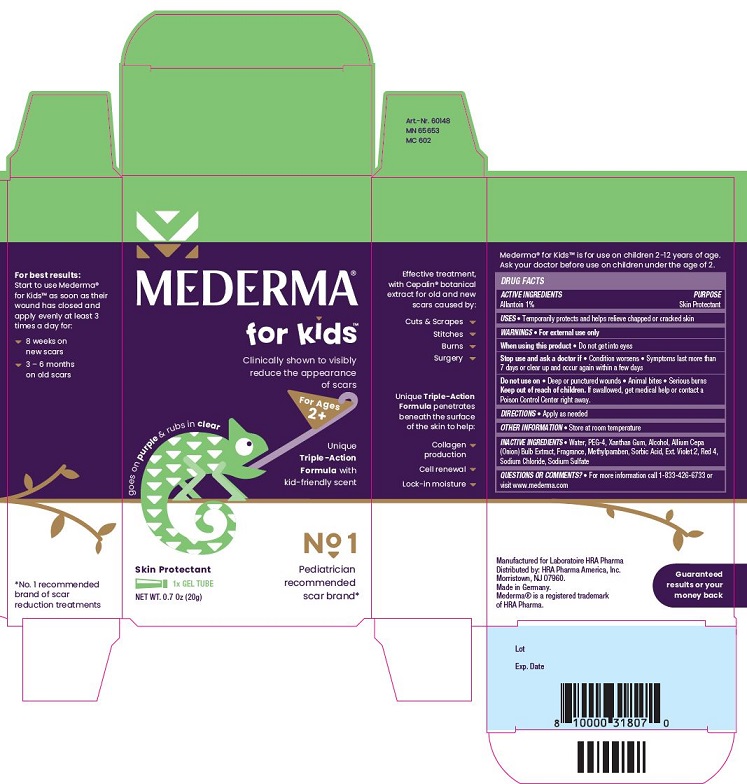
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MEDERMA FOR KIDS
allantoin gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73302-202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Allantoin (UNII: 344S277G0Z) (Allantoin - UNII:344S277G0Z) Allantoin 1 g in 100 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Polyethylene Glycol 200 (UNII: R95B8J264J) Xanthan Gum (UNII: TTV12P4NEE) Alcohol (UNII: 3K9958V90M) Onion (UNII: 492225Q21H) Methylparaben (UNII: A2I8C7HI9T) Sorbic Acid (UNII: X045WJ989B) Ext. D&C Violet No. 2 (UNII: G5UX3K0728) Fd&C Red No. 4 (UNII: X3W0AM1JLX) Sodium Chloride (UNII: 451W47IQ8X) Sodium Sulfate (UNII: 0YPR65R21J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73302-202-20 1 in 1 CARTON 10/01/2020 1 20 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 10/01/2020 Labeler - HRA PHARMA AMERICA, INC. (081160441)