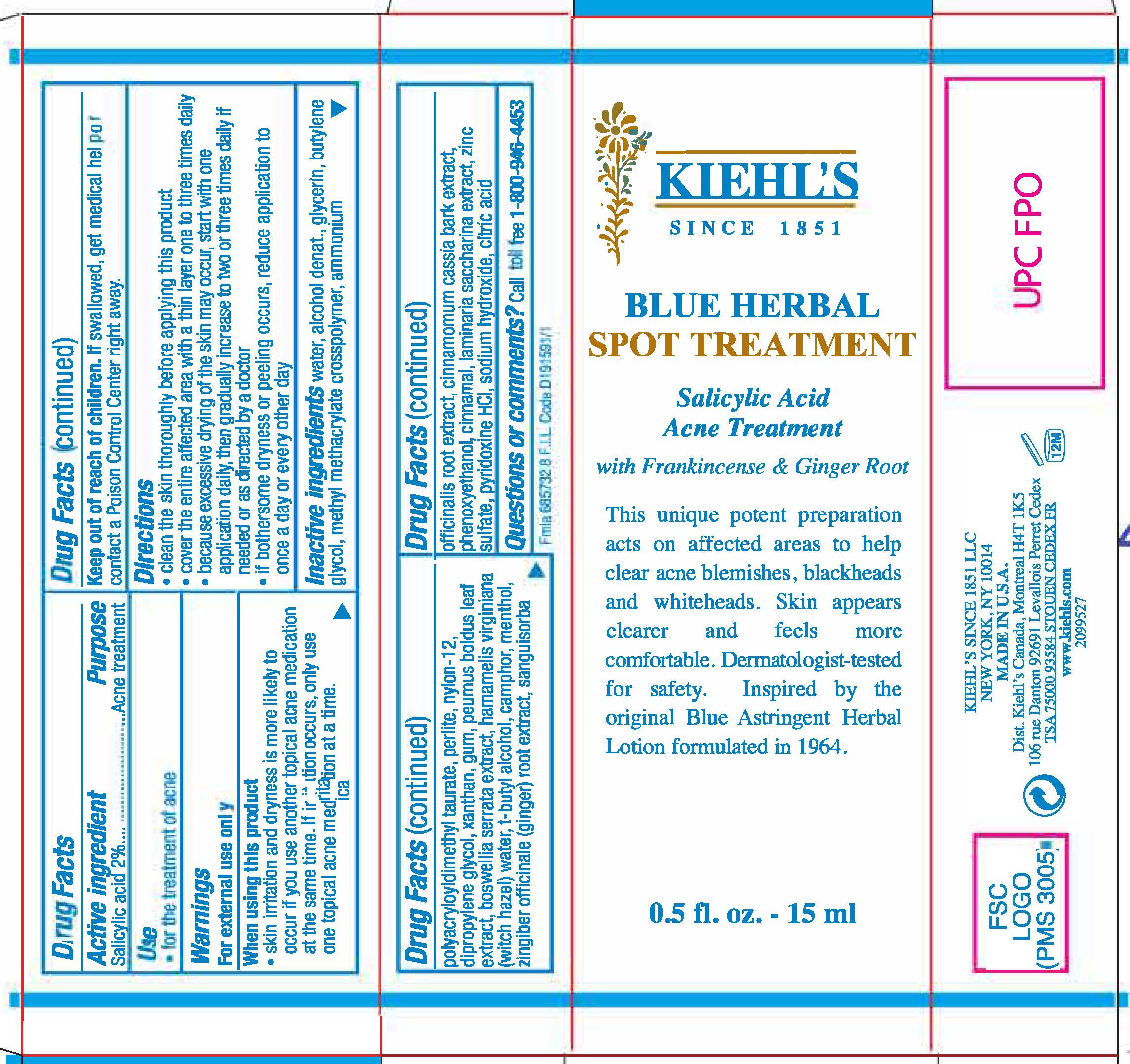
Label: KIEHLS SINCE 1851 BLUE HERBAL ACNE SPOT TREATMENT- salicylic acid lotion
- NDC Code(s): 49967-527-01, 49967-527-02
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
- When using this product
- Keep out of reach of children.
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually ingrease to two or three times dialy if neded or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other dayl
-
Inactive ingredients
water, alcohol denat., glycerin, butylene glycol, methyl methacrylate crosspolymer, ammonium polyacryloyldimethyl taurate, perlite, nylon-12, dipropylene glycol, xanthan gum, peumus boldus leaf extract, boswellia serrata extract, hamamelis virginiana (witch hazel) water, t-butyl alcohol, camphor, menthol, zingiber officinale (ginger) root extract, sanguisorba officinalis root extract, cinnamomum cassia bark extract, phenoxyethanol, cinnamal, laminaria accharina extract, zinc sulfate, pyridoxine HCL, sodium hydroxide, citric acid
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KIEHLS SINCE 1851 BLUE HERBAL ACNE SPOT TREATMENT
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-527 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) AMMONIUM POLYACRYLOYLDIMETHYL TAURATE (55000 MPA.S) (UNII: F01RIY4371) PERLITE (UNII: 0SG101ZGK9) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) XANTHAN GUM (UNII: TTV12P4NEE) PEUMUS BOLDUS LEAF (UNII: Q4EWM09M3O) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) MENTHOL (UNII: L7T10EIP3A) GINGER (UNII: C5529G5JPQ) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) CHINESE CINNAMON (UNII: WS4CQ062KM) PHENOXYETHANOL (UNII: HIE492ZZ3T) CINNAMALDEHYDE (UNII: SR60A3XG0F) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) ZINC SULFATE (UNII: 89DS0H96TB) PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) SODIUM HYDROXIDE (UNII: 55X04QC32I) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-527-01 1 in 1 CARTON 08/31/2016 02/07/2025 1 15 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:49967-527-02 1.5 mL in 1 PACKET; Type 0: Not a Combination Product 08/31/2016 02/07/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/31/2016 02/07/2025 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'Oreal USA, Inc. 185931458 analysis(49967-527) Establishment Name Address ID/FEI Business Operations Cosmetic Essence, LLC dba Voyant Beauty 032565959 manufacture(49967-527) Establishment Name Address ID/FEI Business Operations Paklab 078717086 pack(49967-527)