Label: MIRACLE STEMCELL ALL-IN-ONE- dexpanthenol, niacinamide shampoo
- NDC Code(s): 24765-001-01
- Packager: PHARMACAL-INTERNATIONAL. CO., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
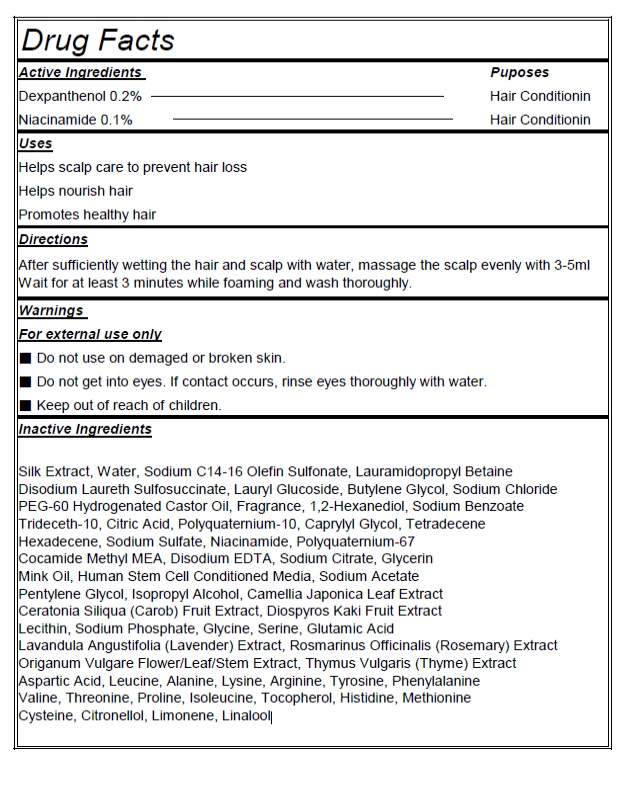
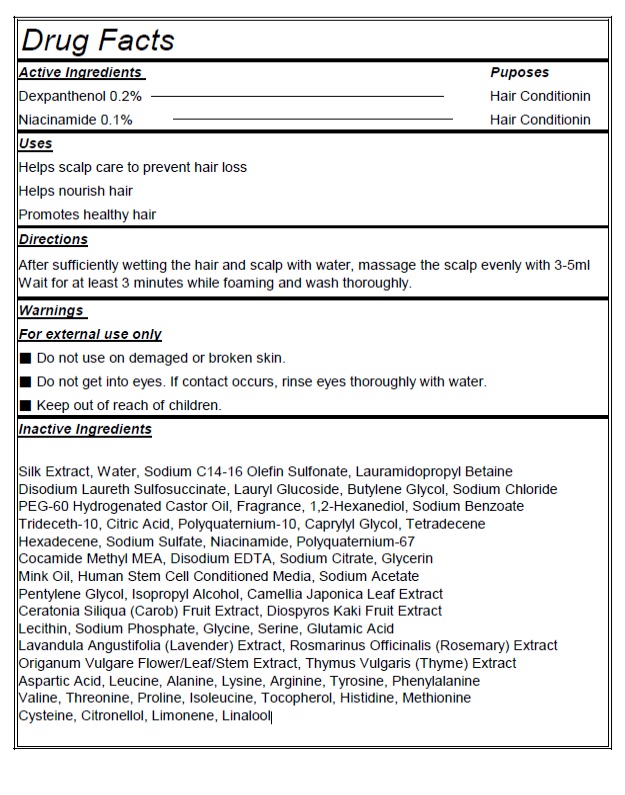
- Actvie Ingrdients Purpose
- PURPOSE
- Indication and Usage
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Inactive Ingredients
Water, Sodium C14-16 Olefin Sulfonate, Lauramidopropyl Betaine, Disodium Laureth Sulfosuccinate, Lauryl Glucoside, Butylene Glycol, Sodium Chloride, PEG-60 Hydrogenated Castor Oil, Fragrance, 1,2-Hexanediol, Sodium Benzoate, Trideceth-10, Citric Acid, Polyquaternium-10, Menthol, Caprylyl Glycol, Tetradecene, Hexadecene, Sodium Sulfate, Cocamide Methyl MEA, Disodium EDTA, Sodium Citrate, Glycerin, Sodium Acetate, Pentylene Glycol, Isopropyl Alcohol, Camellia Japonica Leaf Extract, Ceratonia Siliqua (Carob) Fruit Extract, Diospyros Kaki Fruit Extract, Lecithin, Sodium Phosphate, Glycine, Serine, Glutamic Acid, Lavandula Angustifolia (Lavender) Extract, Rosmarinus Officinalis (Rosemary) Extract, Origanum Vulgare Flower/Leaf/Stem Extract, Thymus Vulgaris (Thyme) Extract, Aspartic Acid, Leucine, Alanine, Lysine, Arginine, Tyrosine, Phenylalanine, Valine, Threonine, Proline, Isoleucine, Tocopherol, Histidine, Methionine, Cysteine, Citronellol, Limonene, Linalool
- Product label
-
INGREDIENTS AND APPEARANCE
MIRACLE STEMCELL ALL-IN-ONE
dexpanthenol, niacinamide shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24765-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXPANTHENOL (UNII: 1O6C93RI7Z) (DEXPANTHENOL - UNII:1O6C93RI7Z) DEXPANTHENOL 0.2 g in 100 g NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength OREGANO FLOWERING TOP (UNII: 7K91ZBK8GJ) WATER (UNII: 059QF0KO0R) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) LAURAMIDOPROPYL BETAINE (UNII: 23D6XVI233) DISODIUM LAURETH SULFOSUCCINATE (UNII: D6DH1DTN7E) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM CHLORIDE (UNII: 451W47IQ8X) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SODIUM BENZOATE (UNII: OJ245FE5EU) TRIDECETH-10 (UNII: G624N6MSBA) CITRIC ACID ACETATE (UNII: DSO12WL7AU) POLYQUATERNIUM-10 (1000 MPA.S AT 2%) (UNII: GMR4PEN8PK) MENTHOL (UNII: L7T10EIP3A) CAPRYLYL GLYCOL (UNII: 00YIU5438U) TETRADECENE (MIXED ISOMERS) (UNII: 5R30W51348) HEXADECENE (MIXED ISOMERS) (UNII: 38H8547VP0) SODIUM SULFATE (UNII: 0YPR65R21J) POLYQUATERNIUM-68 (UNII: R76MNN6P72) COCOYL METHYL MONOETHANOLAMINE (UNII: 79G1T427CF) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) GLYCERIN (UNII: PDC6A3C0OX) SODIUM ACETATE (UNII: 4550K0SC9B) PENTYLENE GLYCOL (UNII: 50C1307PZG) ISOPROPYL ALCOHOL (UNII: ND2M416302) CAMELLIA JAPONICA LEAF (UNII: 4E3VE6KTLY) CAROB (UNII: 5MG5Z946UO) PERSIMMON (UNII: 4V023DD7KL) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM PHOSPHATE (UNII: SE337SVY37) GLYCINE (UNII: TE7660XO1C) SERINE (UNII: 452VLY9402) GLUTAMIC ACID (UNII: 3KX376GY7L) LAVANDULA ANGUSTIFOLIA SUBSP. ANGUSTIFOLIA FLOWERING TOP (UNII: 9YT4B71U8P) ROSEMARY (UNII: IJ67X351P9) THYME (UNII: CW657OBU4N) ASPARTIC ACID (UNII: 30KYC7MIAI) LEUCINE (UNII: GMW67QNF9C) ALANINE (UNII: OF5P57N2ZX) LYSINE (UNII: K3Z4F929H6) ARGININE (UNII: 94ZLA3W45F) TYROSINE (UNII: 42HK56048U) PHENYLALANINE (UNII: 47E5O17Y3R) VALINE (UNII: HG18B9YRS7) THREONINE (UNII: 2ZD004190S) PROLINE (UNII: 9DLQ4CIU6V) ISOLEUCINE (UNII: 04Y7590D77) TOCOPHEROL (UNII: R0ZB2556P8) HISTIDINE (UNII: 4QD397987E) METHIONINE (UNII: AE28F7PNPL) CYSTEINE (UNII: K848JZ4886) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24765-001-01 300 g in 1 BOTTLE; Type 0: Not a Combination Product 12/08/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/08/2023 Labeler - PHARMACAL-INTERNATIONAL. CO., LTD (557805060)