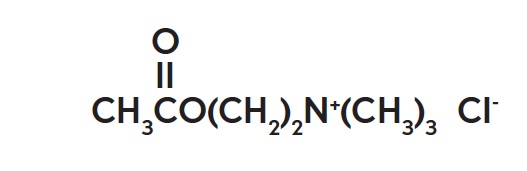Label: MIOCHOL E- acetylcholine chloride kit
- NDC Code(s): 24208-539-20
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
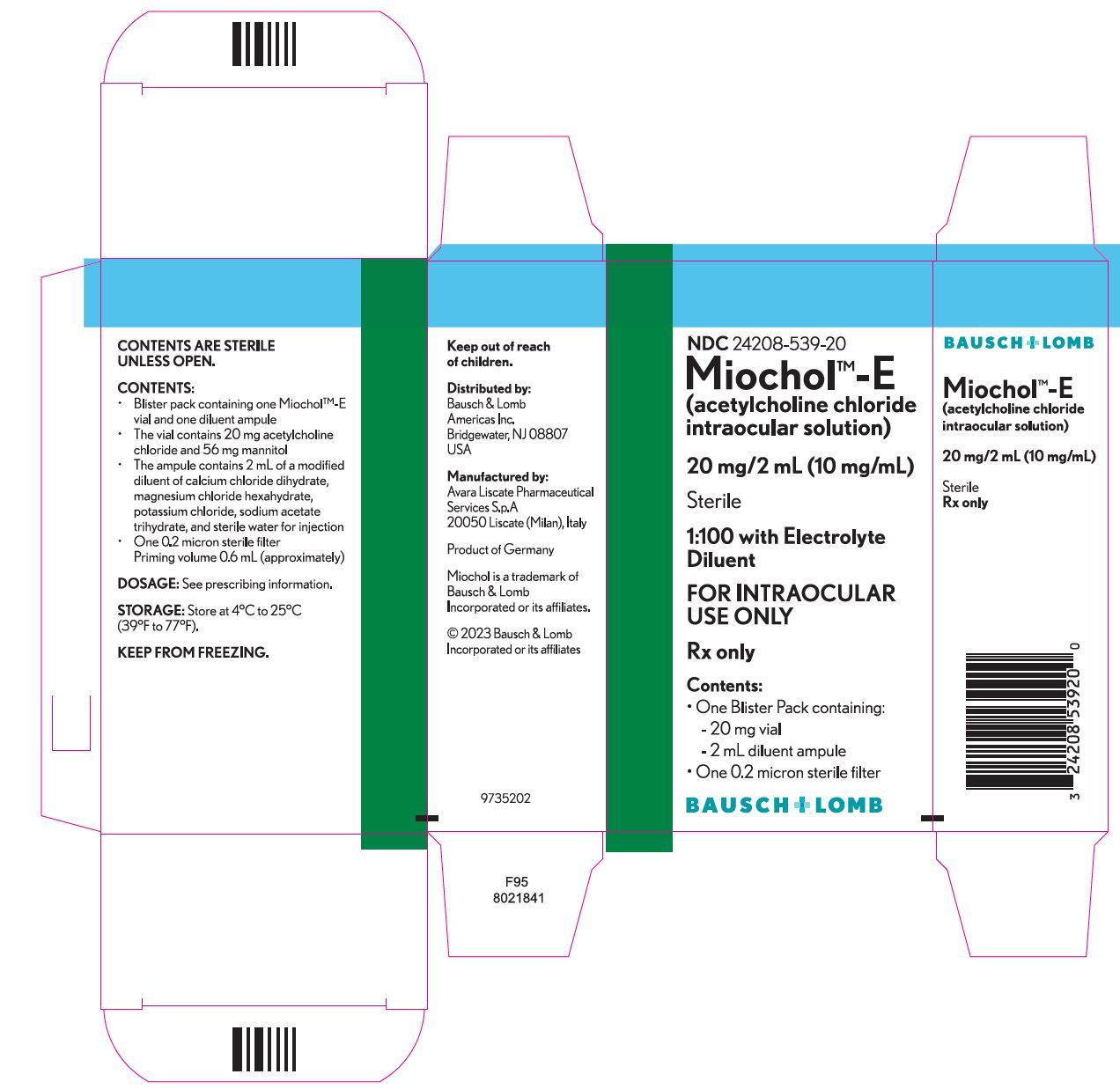
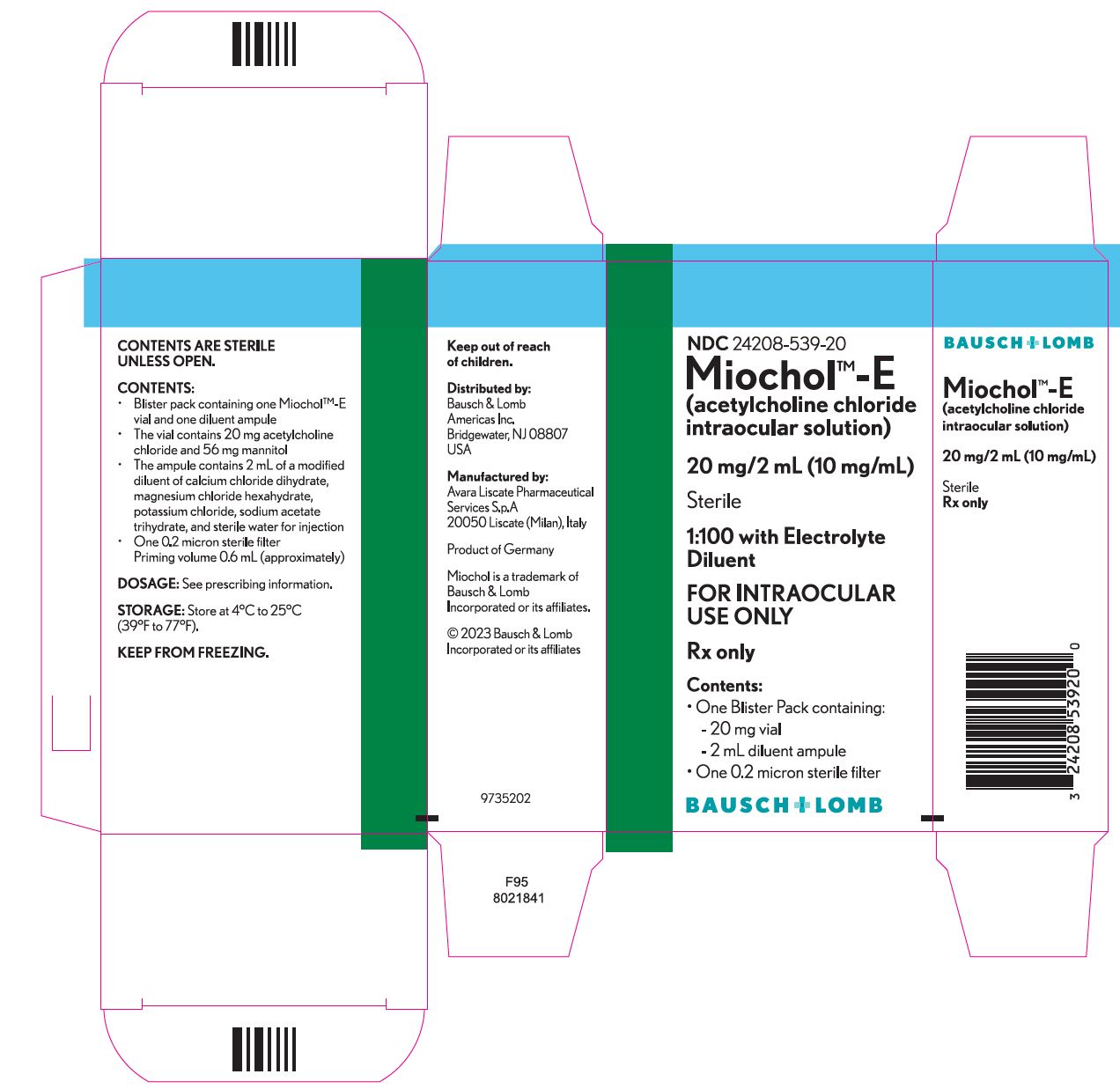
Miochol™-E (acetylcholine chloride intraocular solution) is a parasympathomimetic preparation for intraocular use. It is packaged in a blister pack containing one vial and one diluent ampule. The vial contains 20 mg acetylcholine chloride and 56 mg mannitol. The accompanying ampule contains 2 mL of a modified diluent of calcium chloride dihydrate, magnesium chloride hexahydrate, potassium chloride, sodium acetate trihydrate, and sterile water for injection.
The reconstituted liquid will be a sterile isotonic solution (275–330 milliosmoles/kg) containing 20 mg acetylcholine chloride (1:100 solution) and 2.8% mannitol. The pH range is 5.0–8.2. Mannitol is used in the process of lyophilizing acetylcholine chloride, and is not considered an active ingredient.
The chemical name for acetylcholine chloride, C7H16ClNO2, is Ethanaminium, 2-(acetyloxy)-N,N,N-trimethyl-, chloride and is represented by the following chemical structure:
-
CLINICAL PHARMACOLOGY
Acetylcholine is a naturally occurring neurohormone which mediates nerve impulse transmission at all cholinergic sites involving somatic and autonomic nerves. After release from the nerve ending, acetylcholine is rapidly inactivated by the enzyme acetylcholinesterase by hydrolysis to acetic acid and choline.
Direct application of acetylcholine to the iris will cause rapid miosis of short duration. Topical ocular instillation of acetylcholine to the intact eye causes no discernible response as cholinesterase destroys the molecule more rapidly than it can penetrate the cornea.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
If miosis is to be obtained quickly with Miochol-E, anatomical hindrances to miosis, such as anterior or posterior synechiae, must be released, prior to administration of Miochol-E. During cataract surgery, use Miochol-E only after delivery of the lens.
Aqueous solutions of acetylcholine chloride are unstable. Prepare solution immediately before use. Do not use solution which is not clear and colorless. Discard any solution that has not been used.
Drug Interactions
Although clinical studies with acetylcholine chloride and animal studies with acetylcholine or carbachol revealed no interference, and there is no known pharmacological basis for an interaction, there have been reports that acetylcholine chloride and carbachol have been ineffective when used in patients treated with topical nonsteroidal anti-inflammatory agents.
-
ADVERSE REACTIONS
Infrequent cases of corneal edema, corneal clouding, and corneal decompensation have been reported with the use of intraocular acetylcholine.
Adverse reactions have been reported rarely, which are indicative of systemic absorption. These include bradycardia, hypotension, flushing, breathing difficulties, and sweating.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Miochol™-E (acetylcholine chloride intraocular solution) is instilled into the anterior chamber before or after securing one or more sutures.
Instillation should be gentle and parallel to the iris face and tangential to pupil border.
If there are no mechanical hindrances, the pupil starts to constrict in seconds and the peripheral iris is drawn away from the angle of the anterior chamber. Any anatomical hindrance to miosis must be released to permit the desired effect of the drug. In most cases, 0.5 to 2 mL produces satisfactory miosis. Note that the syringe filter supplied with Miochol-E has a priming volume of 0.6 mL (approximately).
In cataract surgery, use Miochol-E only after delivery of the lens.
Aqueous solutions of acetylcholine chloride are unstable. Prepare solution immediately before use. Do not use solution that is not clear and colorless. Discard any solution that has not been used.
-
DIRECTIONS FOR PREPARING MIOCHOL™-E:
STERILE UNLESS PACKAGE OPEN OR BROKEN
- 1.
- Inspect the unopened blister, vial, and ampule to ensure that they are all intact. Peel open the blister under a sterile field. Maintain sterility of the outer containers of the vial and ampule during preparation of solution.
- 2.
- Aseptically attach a sterile 18–20 gauge, beveled needle to the luer tip of a sterile disposable syringe with a twisting motion to assure a secure fit.
- 3.
- Break open the ampule containing the diluent. The One Point Cut (OPC) ampule must be opened as follows: Hold the bottom part of the ampule with the thumb pointing to the colored dot. Grasp the top of the ampule with the other hand, positioning the thumb at the colored dot, and press back to break at the existing cut under the dot.
- 4.
- Remove the needle protector and withdraw the diluent from the ampule into the syringe. Discard the ampule.
- 5.
- Remove and discard the cap from the top of the vial.
- 6.
- Insert the needle through the center of the vial stopper, and transfer the diluent from the syringe to the vial. Shake gently to dissolve the powder.
- 7.
- Slowly withdraw the solution from the vial through the needle into the syringe. Discard the needle.
- 8.
- Aseptically open the syringe filter pouch, and attach the filter onto the luer tip of the syringe with a twisting motion to assure a secure fit.
- 9.
- Aseptically attach a sterile blunt tip irrigation cannula to the male luer of the filter prior to intraocular irrigation.
Discard the filter appropriately after use.
Do not reuse the syringe filter.
Do not aspirate and inject through the same filter.
-
HOW SUPPLIED
Miochol™-E
(acetylcholine chloride intraocular solution)............................NDC 24208-539-20
One blister pack containing the following components:
- •
- Vial of 20 mg acetylcholine chloride powder for intraocular solution
- •
- Ampule of 2 mL diluent
One 0.2 micron sterile filter
- •
- Priming volume 0.6 mL (approximately)
Storage
Store at 4°C to 25°C (39°F to 77°F).
KEEP FROM FREEZING.
Keep out of reach of children.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Avara Liscate Pharmaceutical Services S.p.A
20050 Liscate (Milan), Italy
Miochol is a trademark of Bausch & Lomb Incorporated or its affiliates.
© 2023 Bausch & Lomb Incorporated or its affiliates
Rev. 03/2023
9735301
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MIOCHOL E
acetylcholine chloride kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:24208-539 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24208-539-20 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/22/1993 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 2 mL Part 2 1 AMPULE 2 mL Part 1 of 2 MIOCHOL E
acetylcholine chloride solutionProduct Information Route of Administration INTRAOCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETYLCHOLINE CHLORIDE (UNII: AF73293C2R) (ACETYLCHOLINE - UNII:N9YNS0M02X) ACETYLCHOLINE CHLORIDE 20 mg in 2 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 56 mg in 2 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020213 09/22/1993 Part 2 of 2 DILUENT
diluent solutionProduct Information Route of Administration INTRAOCULAR Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM ACETATE (UNII: 4550K0SC9B) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020213 09/22/1993 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020213 09/22/1993 Labeler - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Norvatis Pharma Stein AG 488152505 MANUFACTURE(24208-539) Establishment Name Address ID/FEI Business Operations Sanofi S.p.A. 338454274 MANUFACTURE(24208-539) , PACK(24208-539) Establishment Name Address ID/FEI Business Operations Avara Liscate Pharmaceutical Services Spa 564165541 MANUFACTURE(24208-539) , PACK(24208-539)