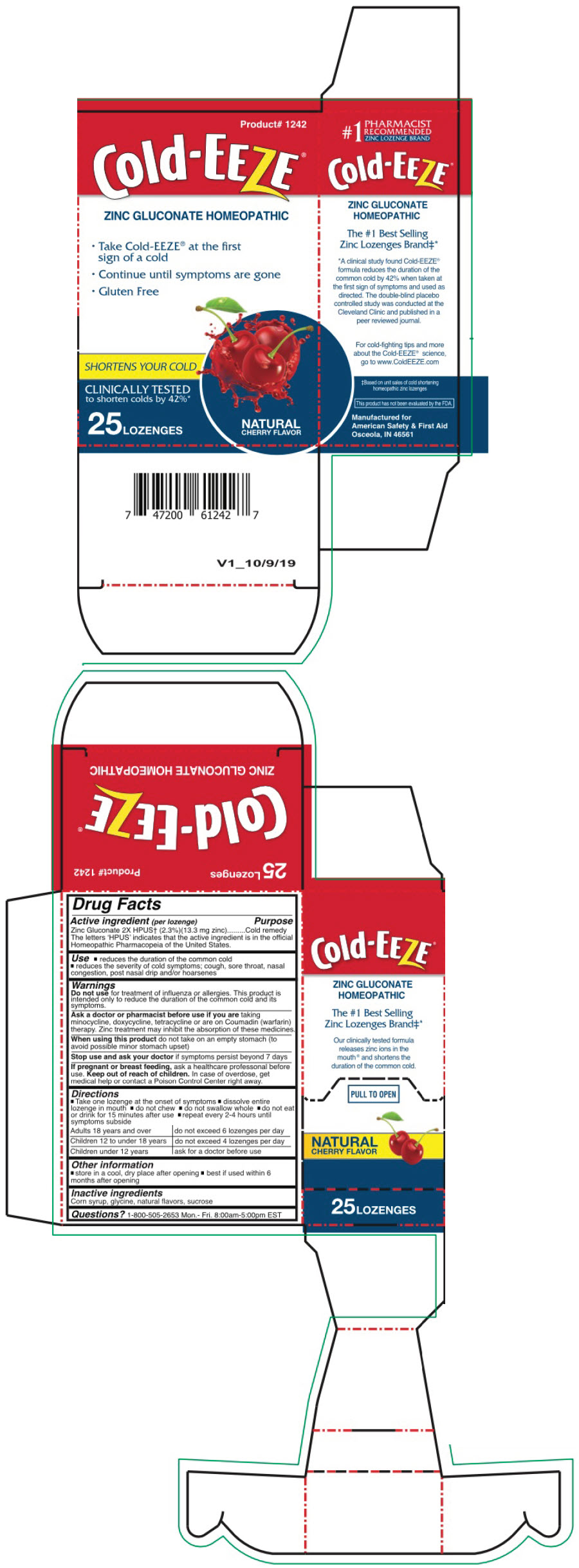
Label: COLD EEZE- zinc gluconate lozenge
-
Contains inactivated NDC Code(s)
NDC Code(s): 73598-1242-1 - Packager: JHK Inc dba American Safety & First Aid
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated September 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient (per lozenge)
- Purpose
- Uses
-
Warnings
Do not use for treatment of influenza or allergies. The product is only intended to reduce the duration of the common cold and its symptoms.
-
Directions
- Take one lozenge at the onset of symptoms
- Dissolve the entire lozenge in the mouth
- Do not chew
- Do not swallow whole
- Do not eat or drink for 15 minutes after use
- Repeat every 2-4 hours until symptoms subside.
Adults 18 years and over - do not exceed 6 lozenges per day
Children 12 - 18 years - do not exceed 4 lozenges per day
Children under 12 years - ask a doctor before use - Other Information
- Inactive Ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 25 Lozenge Box
-
INGREDIENTS AND APPEARANCE
COLD EEZE
zinc gluconate lozengeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73598-1242 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) ZINC GLUCONATE 2 [hp_X] Inactive Ingredients Ingredient Name Strength CORN SYRUP (UNII: 9G5L16BK6N) GLYCINE (UNII: TE7660XO1C) SUCROSE (UNII: C151H8M554) Product Characteristics Color ORANGE Score no score Shape SEMI-CIRCLE Size 24mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73598-1242-1 25 in 1 BOX; Type 0: Not a Combination Product 02/02/2000 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 02/02/2000 Labeler - JHK Inc dba American Safety & First Aid (867236309)