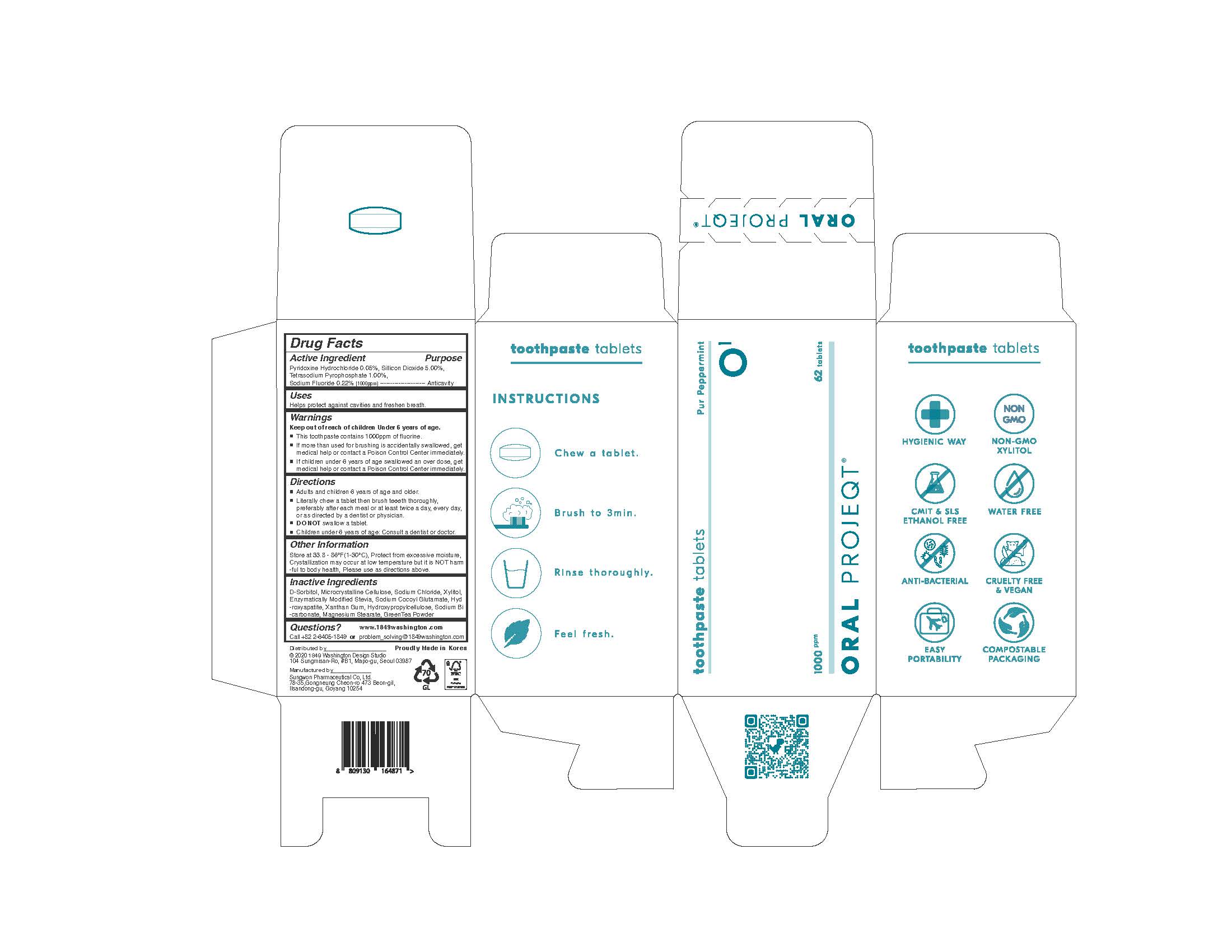
Label: ORAL PROJEQT O1 TOOTHPASTETABLETS- sodium fluoride tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 76058-500-01 - Packager: Sungwon Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
D-Sorbitol, Microcrystalline Cellulose, Sodium Chloride, Xylitol, Enzymatically Modified Stevia, Sodium Cocoyl Glutamate, GreenTea Flavor Powder, Combined Flavor(Coolmint Flavor Powder), Combined Flavor(Peppermint Flavor Powder), L-Menthol, Lemon Juice, Aloe Extract Powder, Tea Extract, Hydroxyapatite, Xanthan Gum, Hydroxypropylcellulose, Sodium Bicarbonate, Magnesium Stearate
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
WARNING
Keep out of reach of children Under 6 years of age.
If more than used for brushing is accidentally swallowed, get
medical help or contact a Poison Control Center immediately.
This toothpaste contains 1000ppm of fluorine.
If children under 6 years of age swallowed an over dose, get
medical help or contact a Poison Control Center immediately. - USES
- INDICATION & USAGE SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ORAL PROJEQT O1 TOOTHPASTETABLETS
sodium fluoride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76058-500 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.22 g SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 5 g SODIUM PYROPHOSPHATE (UNII: O352864B8Z) (PYROPHOSPHORIC ACID - UNII:4E862E7GRQ) SODIUM PYROPHOSPHATE 1 g PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 0.05 g Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) SORBITOL (UNII: 506T60A25R) Product Characteristics Color white Score no score Shape ROUND Size 11mm Flavor Imprint Code None Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76058-500-01 62 in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 08/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/01/2022 Labeler - Sungwon Pharmaceutical Co., Ltd. (689787898) Registrant - Sungwon Pharmaceutical Co., Ltd. (689787898) Establishment Name Address ID/FEI Business Operations Sungwon Pharmaceutical Co., Ltd. 689787898 manufacture(76058-500)