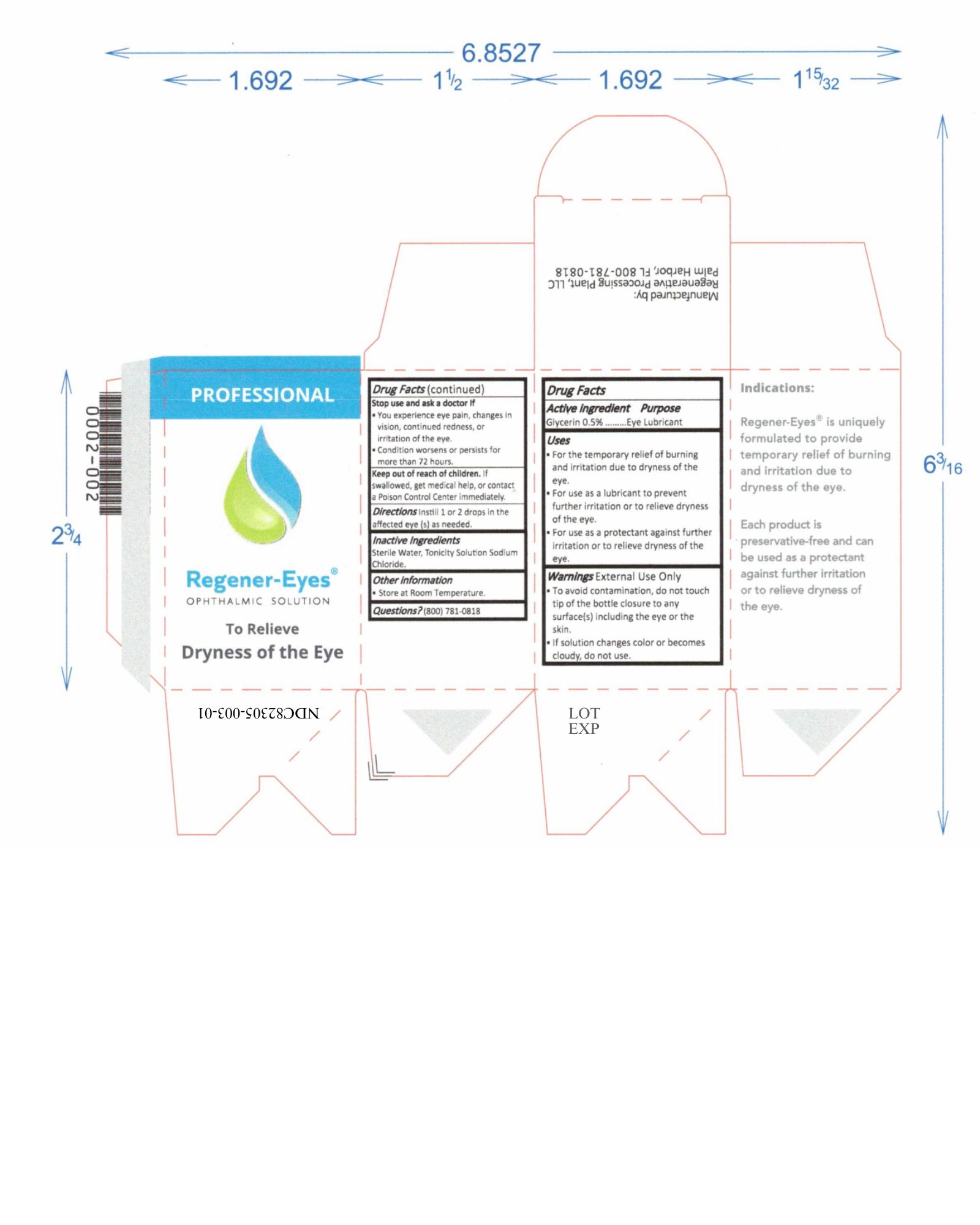
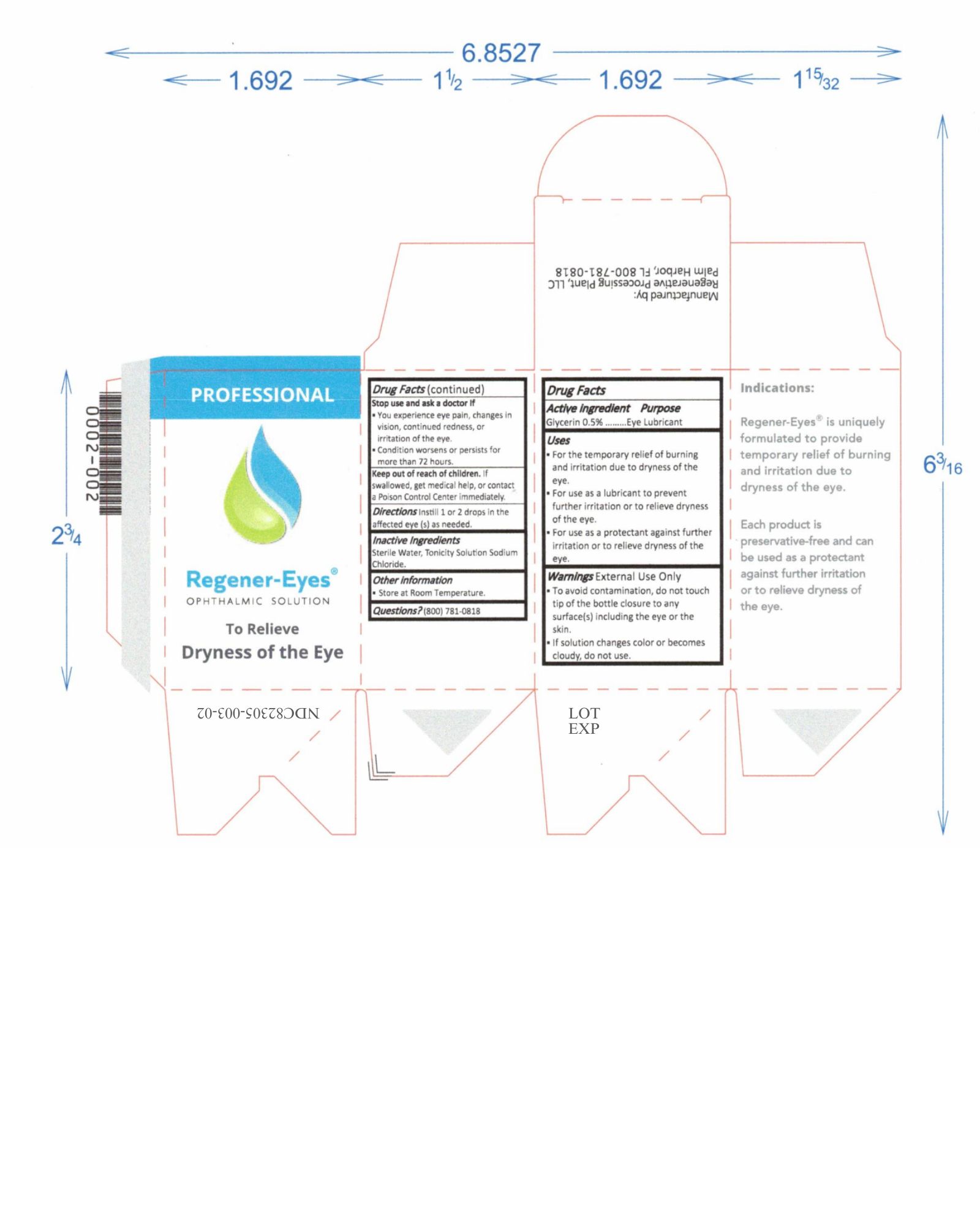
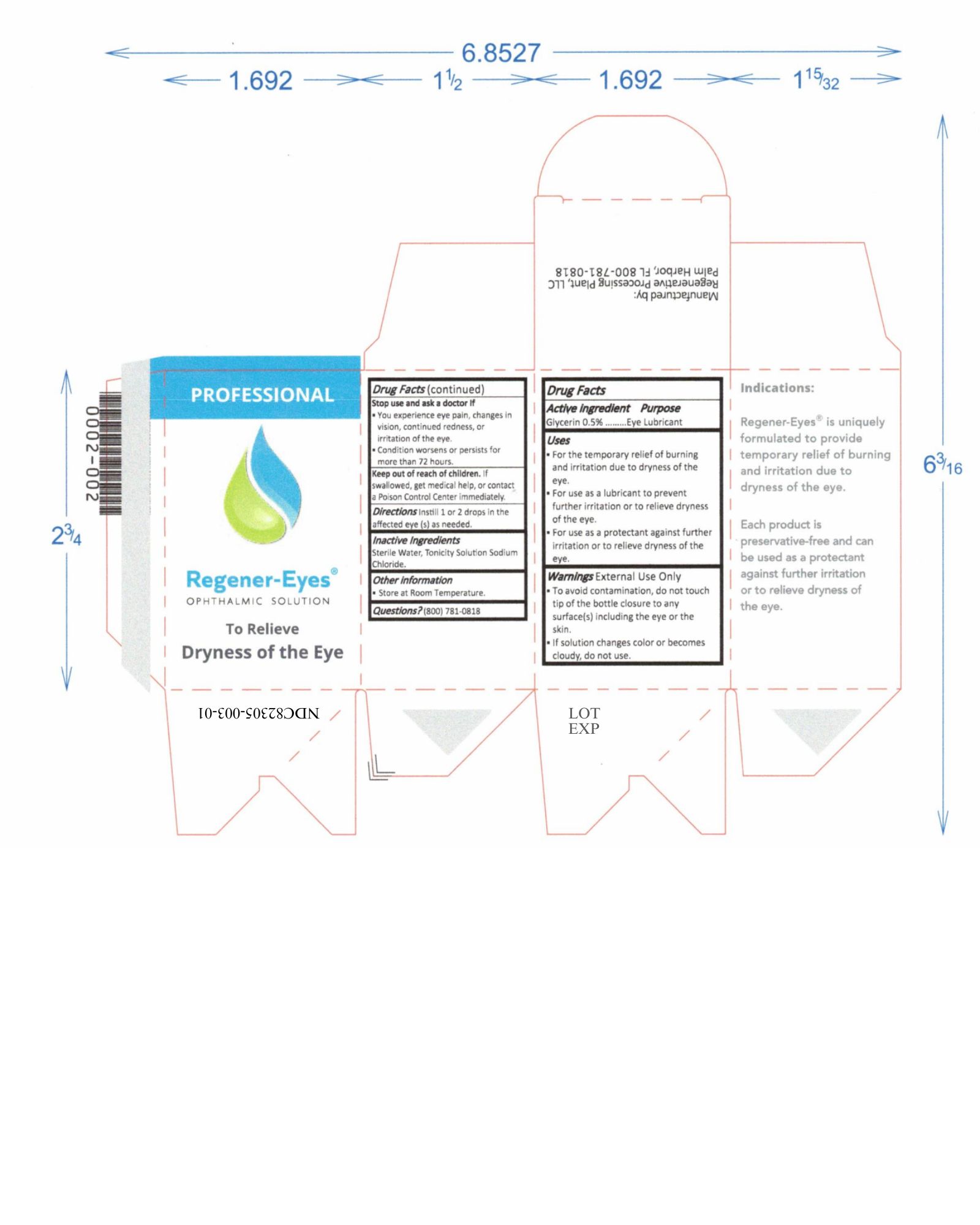
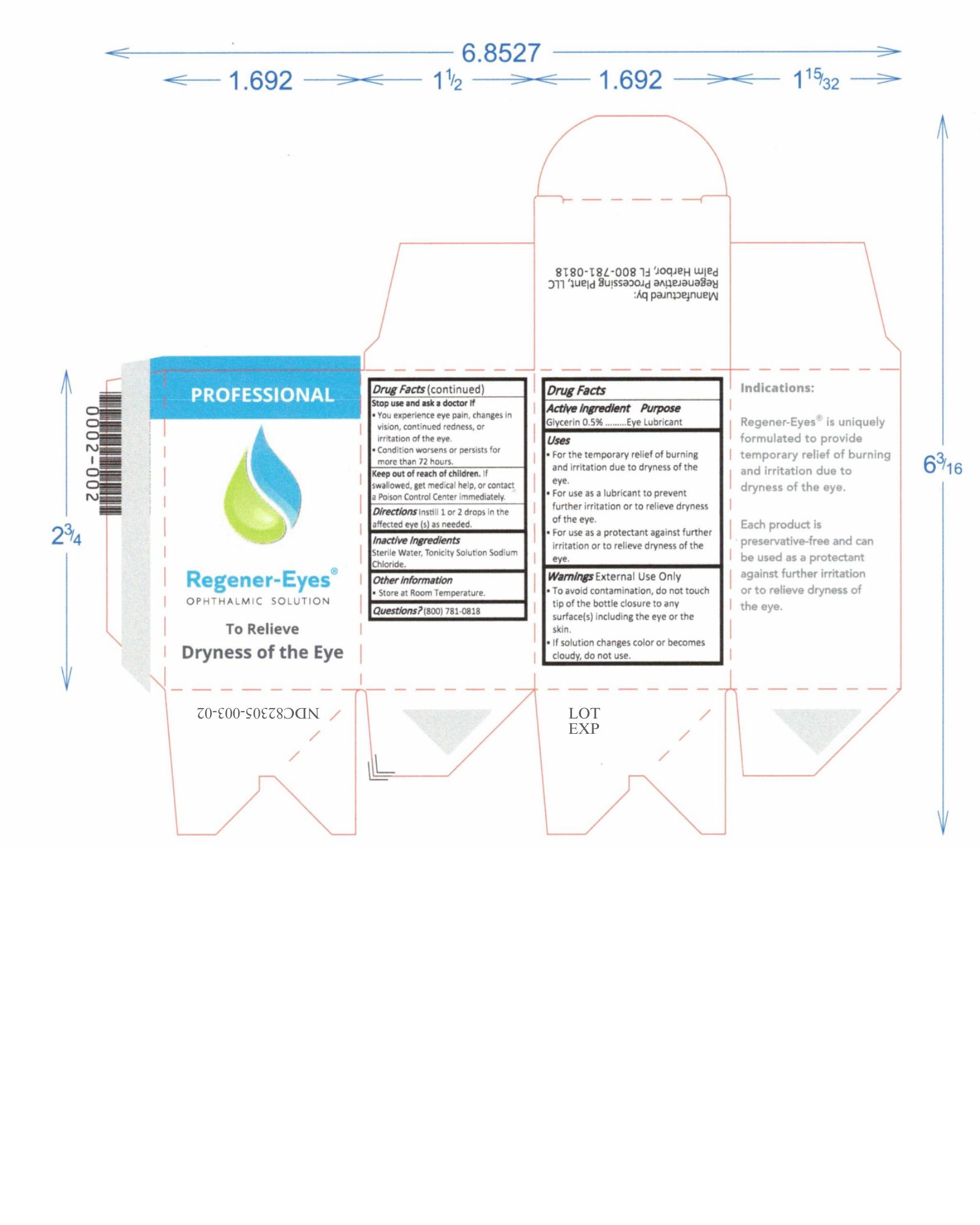
Label: REGENER-EYES PRO- regener-eyes solution/ drops
- NDC Code(s): 82305-003-01, 82305-003-02
- Packager: Regenerative Processing Plant, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
External Use Only
- To avoid contamination, do not touch tip of the bottle closure to any surface(s) including the eye or the skin.
- Do not use if solution changes color or becomes cloudy.
Stop use and ask a doctor If
- You experience eye pain, changes in vision, continued redness, or irritation of the eye.
- Condition worsens or persists for more than 72 hours.
- Keep out of reach of children
- Directions
- Inactive Ingredients
- Other information
- Questions?
- Artwork
-
INGREDIENTS AND APPEARANCE
REGENER-EYES PRO
regener-eyes solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82305-003 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 15 mg in 3 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) 15 mg in 3 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82305-003-01 3 in 1 BOX 08/24/2022 1 3 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:82305-003-02 1 in 1 BOX 08/24/2022 2 3 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 08/24/2022 Labeler - Regenerative Processing Plant, LLC (079446889) Establishment Name Address ID/FEI Business Operations Regenerative Processing Plant 079446889 manufacture(82305-003) , label(82305-003) , pack(82305-003) , analysis(82305-003)