Label: CHAPSTICK TOTAL HYDRATION MOISTURE TINT PRETTY IN PINK- zinc oxide stick
- NDC Code(s): 0573-0873-08, 0573-0873-11
- Packager: Haleon US Holdings LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
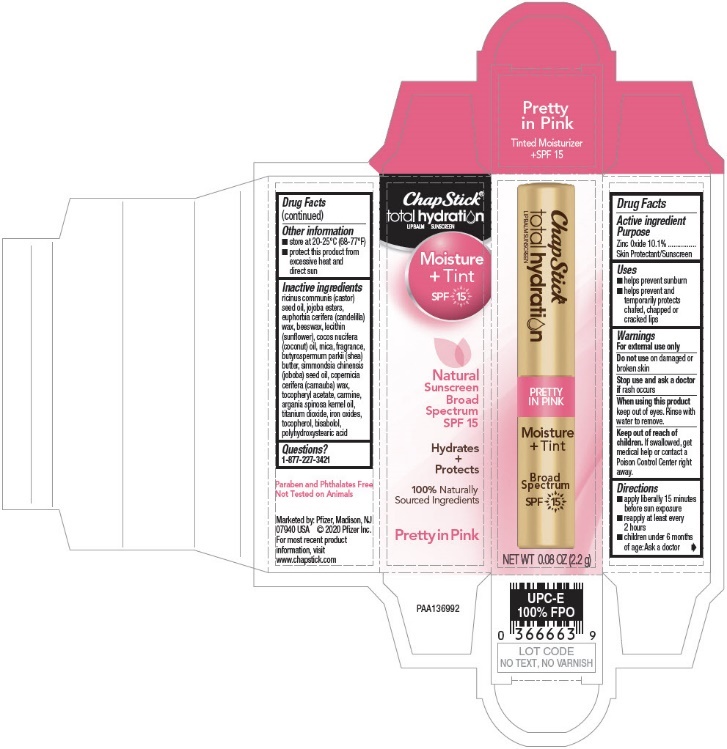
INACTIVE INGREDIENT
Inactive ingredients
ricinus communis (castor) seed oil, jojoba esters, euphorbia cerifera (candelilla) wax, beeswax, lecithin (sunflower), cocos nucifera (coconut) oil, mica, fragrance, butyrospermum parkii (shea) butter, simmondsia chinensis (joboba) seed oil, copernicia cerifera (carnauba) wax, tocopheryl acetate, carmine, argania spinosa kernel oil, titanium dioxide, iron oxides, tocopherol, bisabolol, polyhydroxystearic acid
- QUESTIONS
- PRINCIPAL DISPLAY PANEL - 2.2 g Cylinder Blister Pack
- PRINCIPAL DISPLAY PANEL - 2.2 g Cylinder Carton
-
INGREDIENTS AND APPEARANCE
CHAPSTICK TOTAL HYDRATION MOISTURE TINT PRETTY IN PINK
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0573-0873 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 101 mg in 1 g Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) HYDROGENATED JOJOBA OIL/JOJOBA OIL, RANDOMIZED (IODINE VALUE 57-61) (UNII: 12GIN16K1G) HYDROGENATED JOJOBA OIL/JOJOBA OIL, RANDOMIZED (IODINE VALUE 40-44) (UNII: AS2SZ9757N) CANDELILLA WAX (UNII: WL0328HX19) YELLOW WAX (UNII: 2ZA36H0S2V) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCONUT OIL (UNII: Q9L0O73W7L) MICA (UNII: V8A1AW0880) SHEA BUTTER (UNII: K49155WL9Y) JOJOBA OIL (UNII: 724GKU717M) CARNAUBA WAX (UNII: R12CBM0EIZ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CARMINIC ACID (UNII: CID8Z8N95N) ARGAN OIL (UNII: 4V59G5UW9X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) TOCOPHEROL (UNII: R0ZB2556P8) LEVOMENOL (UNII: 24WE03BX2T) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) Product Characteristics Color PINK, RED Score Shape BULLET (cylindrical) Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0573-0873-08 1 in 1 BLISTER PACK 02/25/2020 1 2.2 g in 1 CYLINDER; Type 0: Not a Combination Product 2 NDC:0573-0873-11 1 in 1 CARTON 03/11/2020 2 2.2 g in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 02/25/2020 Labeler - Haleon US Holdings LLC (079944263)