Label: OCTAPLAS- human plasma proteins solution
- NDC Code(s): 68982-955-01
- Packager: Octapharma USA Inc
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Octaplas safely and effectively. See full prescribing information for Octaplas.
Octaplas, Pooled Plasma (Human), Solvent/Detergent Treated
Solution for Intravenous Infusion
Initial U.S. Approval: 2013INDICATIONS AND USAGE
Octaplas is a solvent/detergent (S/D) treated, pooled human plasma indicated for (2)
• Replacement of multiple coagulation factors in patients with acquired deficiencies (2)
• due to liver disease (2)
• undergoing cardiac surgery or liver transplantation (2)
• Plasma exchange in patients with thrombotic thrombocytopenic purpura (TTP) (2)
DOSAGE AND ADMINISTRATION
For intravenous use only. (3)
Administer Octaplas based on AB0-blood group compatibility. (3)
Indication Dosage Replacement of multiple coagulation factors in patients with acquired deficiencies 10 to 15 milliliters per kg,
Adjust the dose based on the desired clinical responsePlasma exchange in patients with TTP 1 to 1.5 plasma volumes (40 to 60 milliliters per kg) DOSAGE FORMS AND STRENGTHS
Solution for infusion containing 45 to 70 mg human plasma protein per mL in a 200 mL volume. ( 3 )
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
• Transfusion reactions can occur with ABO blood group mismatch ( 5.1 )
• High infusion rates can induce hypervolemia with consequent pulmonary edema or heart failure ( 5.2 )
• Excessive bleeding due to hyperfibrinolysis can occur due to low levels of alpha 2-antiplasmin ( 5.3 )
• Thrombosis can occur due to low levels of Protein S ( 5.4 )
• Citrate toxicity can occur with volumes exceeding one milliliter of Octaplas per kg per minute ( 5.5 )
• Octaplas is made from human blood and may carry the risk of transmitting infectious agents, e.g., viruses and theoretically, the variant Creutzfeldt-Jakob disease and Creutzfeldt-Jakob disease agent ( 5.6 )
ADVERSE REACTIONS
The most common adverse reactions observed in ≥ 1% of patients included pruritis, urticaria, nausea, headache, paresthesia. ( 6 ).
Serious adverse reactions seen in clinical trials were anaphylactic shock, citrate toxicity and hypotension.
To report SUSPECTED ADVERSE REACTIONS, contact Octapharma USA Inc. at phone # 866-766-4860 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Transfusion reactions
5.2 Hypervolemia
5.3 Hyperfibrinolysis
5.4 Thrombosis
5.5 Citrate Toxicity
5.6 Infection Risk from Human Plasma
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- SPL UNCLASSIFIED SECTION
-
1 INDICATIONS AND USAGE
Octaplas is a solvent / detergent (S/D) treated, pooled human plasma indicated for:
- Replacement of multiple coagulation factors in patients with acquired deficiencies
- due to liver disease
- undergoing cardiac surgery and liver transplantation
- Plasma exchange in patients with thrombotic thrombocytopenic purpura (TTP)
-
2 DOSAGE AND ADMINISTRATION
For intravenous use only
Administer Octaplas based on ABO-blood group compatibility.
2.1 Dose
Replacement of coagulation factors in patients with acquired deficiencies due to liver disease or undergoing cardiac surgery or liver transplant:
- Initially infuse of 10 to 15 mL Octaplas per kilogram body weight. This should increase the patient’s plasma coagulation factor levels by approximately 15-25%. If hemostasis is not achieved, use higher doses.
- Adjust dose based on desired clinical response.
- Monitor response, including measurement of activated partial thromboplastin time (aPTT), prothrombin time (PT) and/or specific coagulation factors.
- Plasma exchange in patients with TTP:
- Completely replace plasma volume removed during plasmapheresis with Octaplas. Generally, 1 to 1.5 plasma volumes correspond to 40 to 60 milliliters per kg.[ 1 , 2 ]
2.2 Administration
Administer Octaplas after thawing using an infusion set with a filter.
Octaplas should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if turbid.
Avoid shaking.
Steps for Thawing:
- For water bath:
- Thaw in the outer wrapper in a circulating water bath at +30°C to +37°C (86°F to 98.6°F). An overwrap bag may be used to provide further protection of contents if appropriate.
- Prevent water from contaminating the entry port.
- The minimum thawing time is 30 minutes at 37°C (98.6°F). The thawing time depends on the number of bags in the water bath. If more than one plasma bag is thawed in the same water bath, then the thawing time can be prolonged, but should not exceed 60 minutes.
- For dry tempering system:
- Place the Octaplas bags between the heating plates according to the manufacturer’s instructions.
- Thaw plasma following manufacturer directions between +30°C to +37°C (86°F to 98.6°F). Remove the product when the thawing process is completed. The thawing process may be monitored and recorded using the thawing device printer or barcode scanner recommended by the device manufacturer.
- Monitor the thawing process and record using the thawing device printer or barcode scanner recommended by the device manufacturer.
Do not freeze Octaplas. Discard unused product.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Transfusion reactions
Transfusion reactions can occur with ABO blood group mismatches. Administration of Octaplas must be based on ABO-blood group compatibility.
5.2 Hypervolemia
High infusion rates can induce hypervolemia with consequent pulmonary edema or heart failure. Monitor patients for signs and symptoms of pulmonary edema or heart failure and institute appropriate management.
5.3 Hyperfibrinolysis
Excessive bleeding due to hyperfibrinolysis can occur due to low levels of alpha 2-antiplasmin. Monitor for signs of excessive bleeding in patients undergoing liver transplantation.
5.4 Thrombosis
Thrombosis can occur due to low levels of Protein S. Monitor for signs and symptoms of thrombosis in patients at risk.
5.5 Citrate Toxicity
Citrate toxicity can occur with volumes exceeding one milliliter of Octaplas per kg per minute. The infusion rate should not exceed 0.020-0.025 mmol citrate per kilogram per minute (i.e., less than one milliliter Octaplas per kg per minute). Symptoms attributable to citrate toxicity (hypocalcemia) include fatigue, paresthesia and muscle spasms, especially in patients with liver function disorders. Administer calcium gluconate intravenously into another vein in order to minimize citrate toxicity.
5.6 Infection Risk from Human Plasma
Because Octaplas is made from human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. ALL infections suspected by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Octapharma [1-866-766-4860] or FDA at 1-800-FDA-1088 or www.fea.gov/medwatch. See Description ( 11 )
-
6 ADVERSE REACTIONS
Serious adverse reactions seen in clinical trials were anaphylactic shock, citrate toxicity and severe hypotension.
The most common adverse reactions observed in ≥ 1% of subjects included pruritis, urticaria, nausea, headache and paresthesia.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect rates observed in clinical practice.
Adverse reactions observed in clinical trials derive from 9 clinical trials. The mean dose administered ranged from 6 to 15 milliliters/kg body weight; when used in plasma exchange the dose was between 15 to 75 milliliters/kg. Two of the studies were conducted in healthy volunteers (n=90).
In total, 359 subjects received about 600 transfusion episodes in these trials.
The following table shows the adverse reactions observed in ≥ 1% of subjects in order of severity:
Nervous system disorders
Headache, paresthesiaGastrointestinal disorders
NauseaSkin and subcutaneous tissue disorders
Pruritis, urticaria6.2 Postmarketing Experience
Because post-marketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to product exposure.
Blood system disorders
HyperfibrinolysisImmune system disorders
Hypersensitivity reactions including anaphylactoid and allergic type of reactionsMetabolic and nutritional disorders
AlkalosisCardiovascular disorders
Cardiac arrest, circulatory overload, thromboembolism, tachycardiaRespiratory, thoracic and mediastinal disorders
Respiratory arrest or failure, bronchospasm, pulmonary edema, dyspnea, tachypneaGastrointestinal disorders
Abdominal pain, vomitingSkin and subcutaneous tissue disorders
Rash, erythemaGeneral disorders and administration site conditions
Fever and/or chills, chest discomfort or painInvestigations Seroconversions (passive transfer of antibodies); passive transmission of analytes, e.g. β-human chorionic gonadotropin [ 3 ] Injury, poisoning and procedural complications
Citrate toxicity -
7 DRUG INTERACTIONS
Do not inject drugs containing calcium in the same intravenous line with Octaplas because precipitants may block the line.
Passive transmission of analytes (e.g., β-human chorionic gonadotropin) may result in misleading positive results [ 3 ].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Animal reproduction studies have not been conducted with Octaplas. It is not known whether Octaplas can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Octaplas should be given to a pregnant woman only if clearly needed.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively [ 4 ].
8.4 Pediatric Use
Octaplas was evaluated in 91 pediatric patients (age range 0-20 years) in two post-marketing requirement studies. Patients were dosed based on body weight and doses were adjusted as needed. There were no hyperfibrinolytic or treatment-related thromboembolic events reported by investigators. Please refer to Section 14 for information on clinical studies in the pediatric population.
8.5 Geriatric Use
Clinical studies of Octaplas did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
11 DESCRIPTION
Octaplas is a sterile frozen solution of solvent/detergent (S/D) treated pooled human plasma.
The active ingredient comprises plasma proteins such as albumin, immunoglobulins, other globulins, coagulation factors, complement proteins and protease inhibitors. The content and distribution of plasma proteins in Octaplas are comparable to reference ranges for healthy blood donors, except for Protein S and alpha 2-antiplasmin. Within a mean total protein content of 57 mg/mL, albumin comprises ~50% and immunoglobulin classes G, A, and M comprise ~12%, ~3% and ~1%, respectively. Protein S and alpha 2-antiplasmin, which are labile to S/D treatment, are controlled to ensure levels in the final product of ≥ 0.4 International Units (IU) per mL. Plasma lipids and lipoproteins are reduced due to S/D treatment and subsequent oil and solid phase extraction.
Composition of Octaplas
Component Quantity per 200 mL dose Human plasma proteins 9.0 - 14.0 g Sodium citrate dihydrate 0.88 - 1.48 g Sodium dihydrogen-phosphate dihydrate 0.06 - 0.24 g Glycine 0.80 - 1.20 g Octaplas is manufactured from human plasma collected in US licensed plasma donation centers. All plasma donations are tested for viral markers in compliance with US regulation. In addition, the manufacturing plasma pool may not contain a titer of human Parvovirus B19 DNA exceeding 10.0 IU per microliter and must have a negative result in a test for human Hepatitis E Virus (HEV) RNA by NAT PCR with a sensitivity of ≤ 2.5 log 10 IU/mL.
Each lot of Octaplas is manufactured from pooled plasma of a single ABO blood group (A, B, AB, or O). The manufacturing plasma pool is limited to 390 kg comprising 370-1,520 individual donations/units. Frozen plasma units are thawed and pooled. Sodium dihydrogen phosphate dihydrate is added as a buffer against increase in pH due to loss of CO 2 . After filtration through a 1 µm pore size membrane, the plasma pool is treated with S/D reagents [1% tri(n-butyl) phosphate (TNBP) and 1% Octoxynol for 1-1.5 hours at +30°C (86°F)] to inactivate enveloped viruses. The S/D reagents are removed by sequential oil and solid phase extraction procedures. Glycine is added to adjust the osmolality. Plasma with glycine is applied to a column filled with affinity ligand resin intended for selective binding of prion protein (PrP Sc ). The effectiveness of this step in removal of prion infectivity from the product has not been established. After sterile filtration, the product is filled into sterile polyvinyl chloride blood bags, labeled, deep-frozen and stored at a temperature of ≤ -18°C (-0.4°F). The finished product is tested for coagulation factors II, V, VII, VIII, IX, X and XI, Protein C, Protein S, alpha 2-antiplasmin, fibrinogen and ADAMTS13.
The S/D treatment step has been validated to effectively inactivate relevant pathogenic and model enveloped viruses as summarized in Table 1.
Table 1 Virus Reduction During Octaplas Manufacture
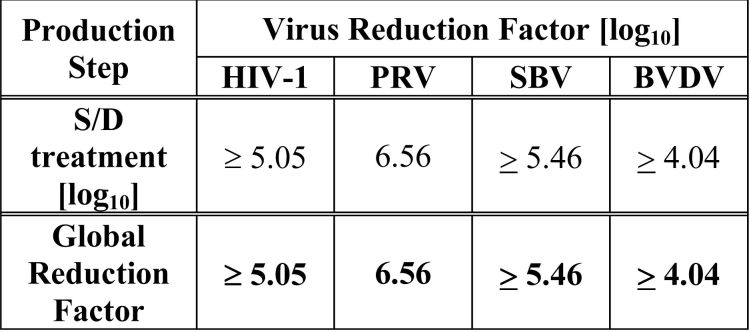
HIV-1: Human Immunodeficiency Virus – 1
PRV: Pseudorabies Virus
SBV: Sindbis Virus
BVDV: Bovine Viral Diarrhea Virus
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Coagulation factor activities in the final product are controlled to obtain levels within the range of normal human plasma. Protein S and alpha 2-antiplasmin, which are labile to S/D treatment, are controlled to ensure levels in the final product of ≥ 0.4 International Units (IU) per mL.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
TNBP and Octoxynol used in the manufacturing process for viral inactivation may be present in the final product at levels not exceeding 2.0 µg/mL for TNBP and 5.0 µg/mL for Octoxynol.
Toxicity
No evidence of toxicity was observed for TNBP + Octoxynol in sub-acute toxicity studies. [ 5 ]
Mutagenicity
No evidence of mutagenicity was observed in in vitro or in vivo mutagenicity studies performed for TNBP. [ 6 - 11 ]
-
14 CLINICAL STUDIES
The Octaplas predecessor product was studied in healthy volunteers and in subjects with liver disease, liver transplantation, cardiac surgery and TTP.
A prospective, open-label, parallel group, non-randomized study was performed in surgical patients who were allocated to receive either a single infusion of Octaplas (N=20) or no plasma treatment (N=26) during open heart surgery.[ 12 ] A historical control group of patients who received standard single-donor FFP (N=20) was used to compare safety and efficacy. The average dose of Octaplas was 700 mL (range 200 to 3400 mL), compared with 1012 mL (range 500 to 4000 mL) for standard FFP. The choice of plasma product (Octaplas or FFP) did not appear to influence the postoperative course with respect to volume of postoperative bleeding, need for reoperation secondary to bleeding or length of postoperative hospital stay. This study was not powered to detect a difference in efficacy.
A prospective, single-blind, randomized study investigated the safety and efficacy of Octaplas compared with standard FFP in adult patients (N=55) with coagulopathy due to liver disease (LD) or liver transplantation (LTX), and for management of newly diagnosed thrombotic thrombocytopenic purpura (TTP).[ 13 - 15 ] Octaplas was infused in 13 of 24 patients with LD, 12 of 25 patients with LTX and all 3 patients with TTP. Within the LD and the LTX groups, patients were comparable in all clinical aspects and in the dose of plasma given. There were no relevant changes in any coagulation factors, but Protein C and fibrinogen improved in both groups, accompanied by a corresponding improvement in partial thromboplastin time (PTT) levels 24 hours after infusion. Similar degrees of correction of prolonged international normalized ratio (INR) and PTT values were achieved with both Octaplas and FFP. All 3 patients with TTP attained platelet counts of >50 x 10 9 /L by Day 10. This study was not powered to detect a difference in efficacy.
A prospective, open label, non-randomized study was conducted in postoperative open heart surgery patients (N=67) admitted to the surgical intensive care unit who were in need of plasma transfusion for acute bleeding or were at risk of bleeding; 36 patients received Octaplas (600 mL) and 31 received FFP (600 mL).[ 16 ] Measured parameters included PT, PTT, free Protein S and alpha 2-antiplasmin measured before treatment and 60 minutes after termination of plasma infusion. The decrease in PT and PTT and the rise in free Protein S were similar between the two study arms. Alpha 2-antiplasmin declined after Octaplas and remained unaffected by FFP. Clinical hemostasis evaluations were similar between the two treatment regimens. This study was not powered to detect a difference in efficacy.
A prospective, open-label, randomized, controlled, cross-over study in two groups (N=30 each) of healthy adult volunteers (mean age: 32.6±9.1 years) was carried out to show the relative recovery of coagulation factors and other hemostatic parameters after infusion of 1200 mL of Octaplas or the predecessor product, following a standard plasmapheresis of 600 mL. Coagulation factors (FI, FII, FV, FVII, FVIII, FIX, FX, and FXI) and hemostatic parameters (aPTT, PT and Protein C) were assessed post-infusion at 15 minutes, 2 hours and 24 hours. The primary objective was to demonstrate equivalence for recovery using a 10% margin. All coagulation and hemostatic parameters met the equivalence criterion. To verify the assumption of improvement in alpha 2-antiplasmin concentrations, a test for superiority was conducted. Statistically significant differences favoring Octaplas were found at 15 minutes (P=0.0012) and 2 hours (P=0.0190) post-transfusion in the per protocol population. Increased plasma levels of alpha 2-antiplasmin after infusion of Octaplas compared with the predecessor product may result from increased concentrations of this plasmin inhibitor found in Octaplas.
A prospective, open-label, multicenter, single arm, postmarketing study was conducted to investigate the safety, tolerability and efficacy of Octaplas in the management of pediatric patients requiring replacement of multiple coagulation factors. A total of 50 patients [37 neonates/infants (0 to 2 years old) and 13 children and adolescents (> 2 to 16 years old)] requiring cardiac surgery (N=40), liver transplantation and/or with liver dysfunction (N=5), sepsis-related coagulopathy (N=4) and hypoxic encephalopathy (N=1) were included in the study. There were no hyperfibrinolytic or treatment-related thromboembolic events reported by investigators. The investigators’ overall safety assessment was prospectively defined as excellent (treatment was well tolerated by the patient), moderate (adverse reactions were observed, but easily resolved or not clinically significant) or poor (adverse reactions were observed requiring significant medical intervention) to describe the patient’s experience with Octaplas. Overall safety was assessed by investigators as ”excellent” for all 50 patients. Hemostatic parameters as measured by INR, PT, aPTT, thromboelastography (TEG) or thromboelastometry (TEM) were within expected ranges following use of Octaplas.
A prospective, open-label, multicenter, single-arm, postmarketing study assessed the safety and tolerability of Octaplas in the management of subjects who underwent therapeutic plasma exchange (TPE). A total of 102 TPE procedures were performed in 41 subjects aged 2 to 20 years [young children: Group 1 (age 2 to <12): N=15; adolescents: Group 2 (12 to <17): N=13; and young adults: Group 3 (≥17): N=13]. Overall, a total of 135,137 mL of Octaplas was administered. The underlying disease category for these subjects included immune system disorders (N=14), nervous system disorders (N=12), renal and urinary disorders (N=8), infections and infestations (N=4), and other disorders (N=3). Each subject underwent between 1 and 6 TPEs (mean: 2.5 TPEs). The actual dose administered per TPE ranged from 4 mL/kg to 72 mL/kg (mean: 28.6 mL/kg), with a mean total volume administered per TPE of 1325 mL (range: 113 mL to 4000 mL). Infusion rates (mean) were similar across age groups (0.32 mL/kg/min to 0.41 mL/kg/min). No thrombotic or thromboembolic events were found in any study subject. In total, 8 adverse reactions were found in 4 subjects (9.8%). These included 5 adverse reactions in three adolescents (4 in TPE #1 and 1 in TPE #4) and 3 adverse reactions in one young adult (2 in TPE #1 and 1 in TPE #2). Adverse reactions in adolescents included citrate toxicity (2 in 2 subjects), and headache, pyrexia, and urticaria (each reported in 1 subject), while adverse reactions found in young adults included inflammatory marker (C-reactive protein, procalcitonin) increased, myalgia, and nausea (each reported in 1 subject). Most (7/8) adverse reactions were mild in intensity and recovered/resolved by end of study. No treatment-related serious adverse events were reported. Overall safety was assessed by investigators as excellent for most subjects (>90%) at 24 hours after each TPE throughout the study using prespecified definitions of excellent, good and poor.
-
15 REFERENCES
- Hellstern, P., et al. "Practical guidelines for the clinical use of plasma." Thromb Res. 107 Suppl 1 (2002): S53-S57.
- Scully, M., et al. "Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies." British Journal of Haematology 158.3 (2012): 323-35.
- Jilma-Stohlawetz P, et al. "False-positive pregnancy test after transfusion of solvent/detergent-treated plasma. " Transfusion 57.12 (2017): 2965–8.
- FDA Briefing Document Risk Communication Advisory Committee Meeting March 5-6, 2018. Communicating Information about Risks in Pregnancy in Product Labeling for Patients and Providers to Make Informed Decisions about the Use of Drugs during Pregnancy
- Horowitz B: Potential accumulation of tri(n-butyl)phosphate in solvent-detergent virus-inactivated plasma products. Transfusion 1991;31:871
- Hanna PJ, Dyer KF: Mutagenicity of organophosphorus compounds in bacteria and Drosophila. Mutat.Res. 1975;28:405-420
- Batt KJ, Healy CE, Kneiss JJ, et al: Genotoxicity testing of tributyl phosphate. – 1992
- Wangenheim J, Bolcsfoldi G: Mouse lymphoma L5178Y thymidine kinase locus assay of 50 compounds. Mutagenesis 1988;3:193-205
- Assinder SJ, Upshall A: Paramorphogenic and genotoxic activity of Triton X-100 and sodium dodecyl sulphate in Aspergillus nidulans. Mutat.Res. 1985;142:179-181
- Buttar HS, Swierenga SH, Matula TI: Evaluation of the cytotoxicity and genotoxicity of the spermicides nonoxynol-9 and octoxynol-9. Toxicol.Lett. 1986;31:65-73
- Auletta CS, Kotkoskie LA, Saulog T, et al: A dietary oncogenicity study of tributyl phosphate in the CD-1 mouse. Toxicology 1998;128:135-14
- Solheim, B. G. et al. "The Use of OCTAPLAS in Patients Undergoing Open Heart Surgery." DIC: Pathogenesis, Diagnosis and Therapy of Disseminated Intravascular Fibrin Formation. Ed. G. Müller-Berghaus and et al. Elsevier Science Publishers B.V., Netherlands, 1993. 253-62.
- Evans, G., et al. "Solvent/detergent fresh frozen plasma as primary treatment of acute thrombotic thrombocytopenic purpura." Clin Lab Haematol 21.2 (1999): 119-23.
- Freeman, J. W., et al. "A randomized trial of solvent/detergent and standard fresh frozen plasma in the treatment of the coagulopathy seen during Orthotopic Liver Transplantation." Vox Sang 74 Suppl 1 (1998): 225-29.
- Williamson, L. M., et al. "A randomized trial of solvent/detergent-treated and standard fresh-frozen plasma in the coagulopathy of liver disease and liver transplantation." Transfusion 39.11-12 (1999): 1227-34.
- Haubelt, H., et al. "Effects of solvent/detergent-treated plasma and fresh-frozen plasma on haemostasis and fibrinolysis in complex coagulopathy following open- heart surgery." Vox Sang 82.1 (2002): 9-14.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Octaplas is supplied in polyvinyl chloride blood bags containing 200 mL frozen solution and has a slightly yellow appearance.
NDC Number Blood group 68982-952 - 01 Blood group A 68982-953 - 01 Blood group B 68982-954 - 01 Blood group AB 68982-955 - 01 Blood group 0
- Store at ≤ -18°C (-0.4°F) for 3 years from the date of manufacture.
- Store protected from light.
- Thaw product according to instructions in section 2.2.
- Use thawed product within 24 hr. if stored at 1 - 6°C (33.8°F to 42.8°F) or within 8 hr. if stored at 20 - 25°C (68°F to 77°F).
- Do not refreeze thawed product.
- Do not use product that is cloudy or has deposits.
- Discard product after the expiration date printed on the container label.
-
17 PATIENT COUNSELING INFORMATION
Inform patients to report:
- Early signs of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, or anaphylaxis.
- Development of edema or volume overload including shortness of breath or breathing difficulties
Remind patients that Octaplas is made from human blood and may contain infectious agents that can cause disease. Report flu-like or other symptoms or viral infection.
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE- 112 75, Sweden
U.S. License No. 1646
Distributed by:
Octapharma USA Inc.
117 West Century Road
Paramus, NJ 07652
- 18 PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OCTAPLAS
human plasma proteins solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:68982-955 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PLASMA PROTEIN FRACTION (HUMAN) (UNII: 6D53G0FD0Z) (PLASMA PROTEIN FRACTION (HUMAN) - UNII:6D53G0FD0Z) PLASMA PROTEIN FRACTION (HUMAN) 11.5 g in 200 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68982-955-01 200 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125416 07/18/2013 Labeler - Octapharma USA Inc (606121163)


