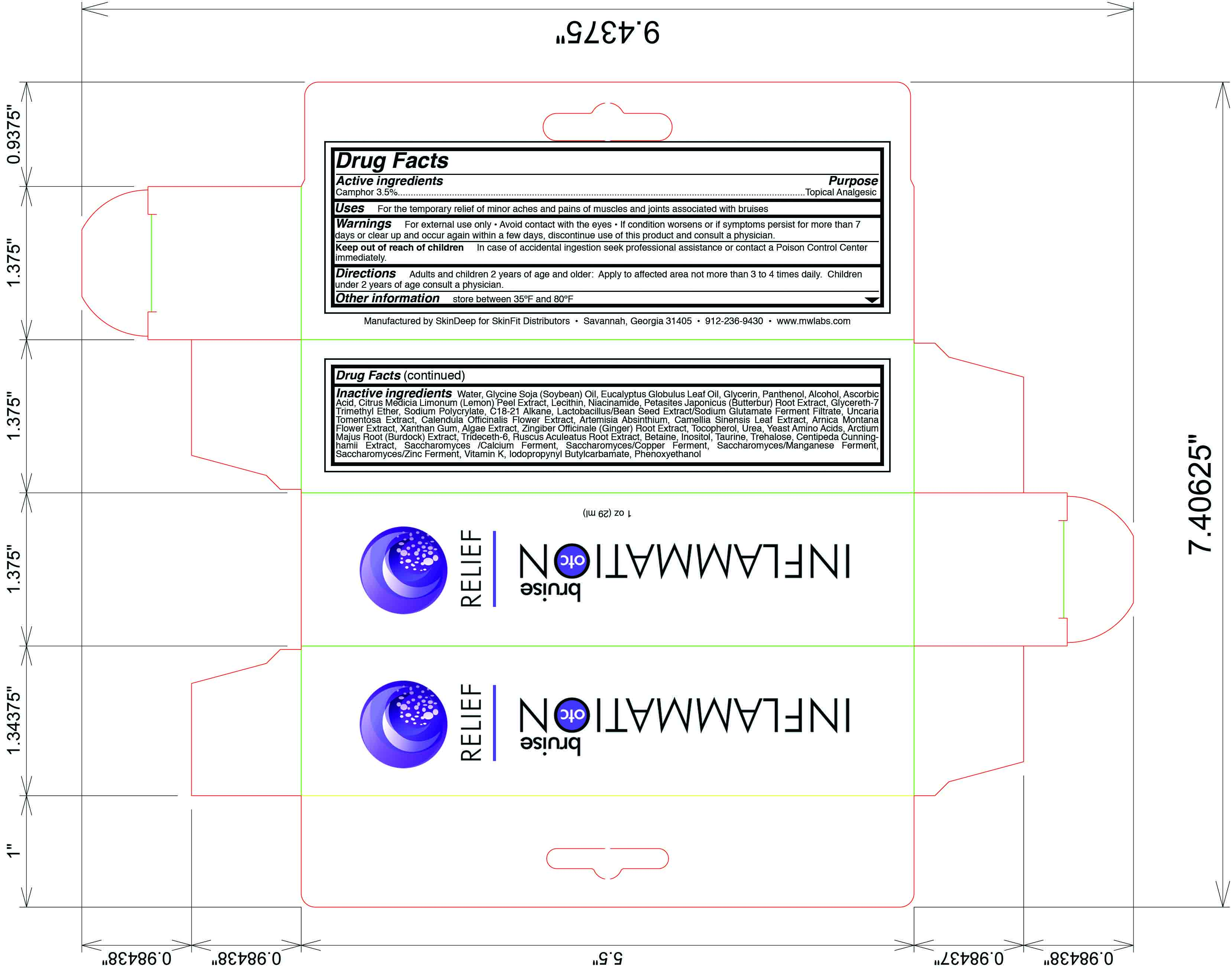
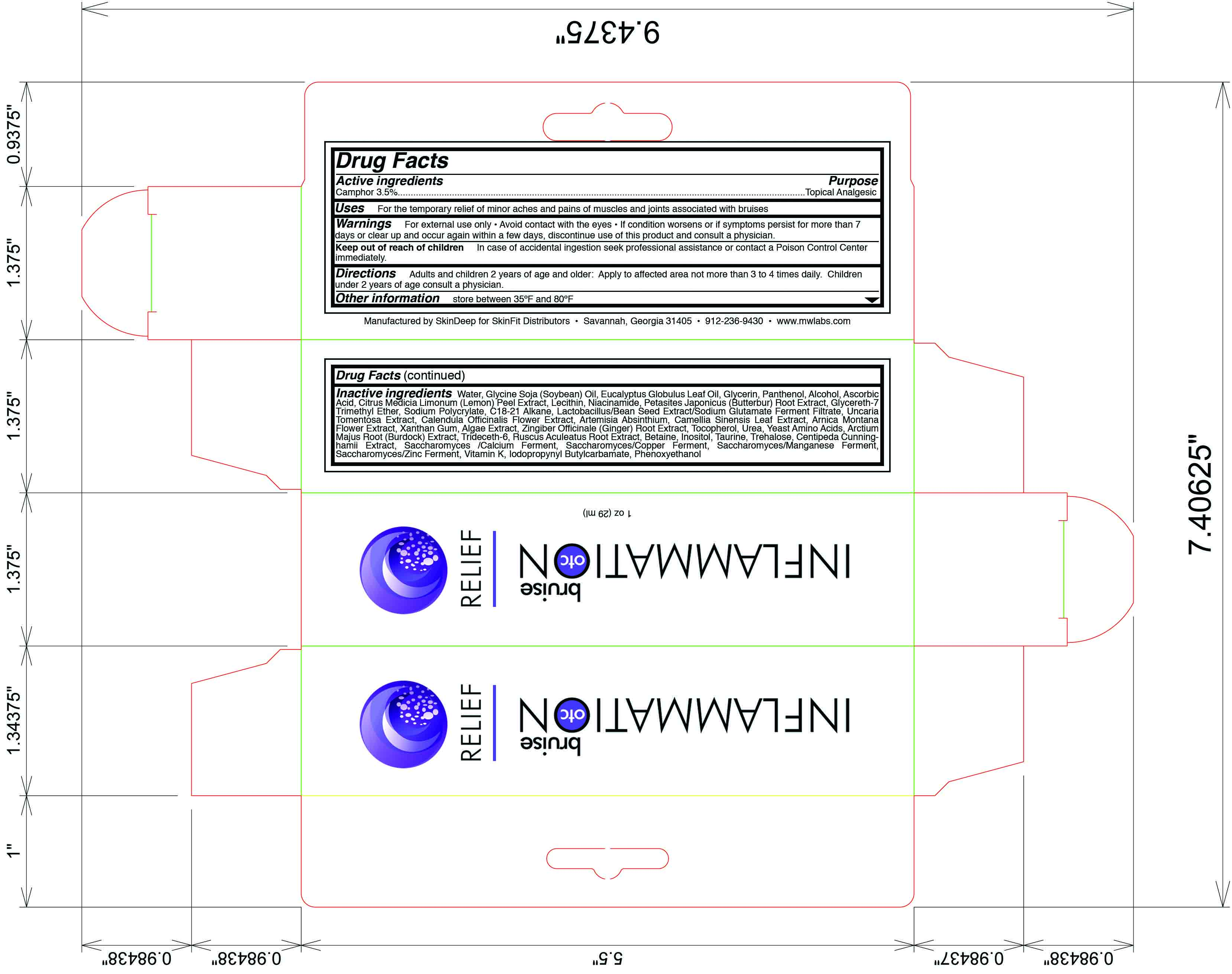
Label: INFLAMMATION OTC BRUISE RELIEF- camphor lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 45156-5502-1, 45156-5502-2 - Packager: Skin Deep
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 8, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warning
- Keep out of reach of children
- Directions
-
Inactive Ingredients
water, glycine soja (soybean) oil, glycerin, panthenol, alcohol, ascorbic acid, citrus medica limonum (lemon) peel extract, niacinamide, petasites japonicus (butterbur) root extract, glycereth-7 trimethyl ether, sodium polycrylate, c18-21 alkane, lactobacillus/bean seed extract/sodium glutamate ferment filtrate, uncaria tomentosa extract, calendula officinale (ginger) root extract, tocopherol, urea, yeast amino acid, arctium majus root (burdock) extract, trideceth-6, ruscus aculeatus root extract, inositol, taurine, trehalose, centipeda cunninghamii extract, saccharomyces/calcium ferment, saccharomyces/copper ferment, saccharomyces/manganese ferment, saccaromyces/zinc ferment, vitamin k, iodopropynyl butylcarbamate, phenoxyethanol
- Questions and Comments
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
INFLAMMATION OTC BRUISE RELIEF
camphor lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45156-5502 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Camphor (Natural) (UNII: N20HL7Q941) (Camphor (Natural) - UNII:N20HL7Q941) Camphor (Natural) 32 g in 1000 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Alcohol (UNII: 3K9958V90M) Dexpanthenol (UNII: 1O6C93RI7Z) Soybean Oil (UNII: 241ATL177A) Ascorbic Acid (UNII: PQ6CK8PD0R) Lemon Oil (UNII: I9GRO824LL) Lecithin, Soybean (UNII: 1DI56QDM62) Niacinamide (UNII: 25X51I8RD4) Cat's Claw (UNII: 9060PRM18Q) Calendula Officinalis Flower (UNII: P0M7O4Y7YD) Wormwood (UNII: F84709P2XV) Green Tea Leaf (UNII: W2ZU1RY8B0) Arnica Montana Flower (UNII: OZ0E5Y15PZ) Xanthan Gum (UNII: TTV12P4NEE) Carrageenan (UNII: 5C69YCD2YJ) Ginger (UNII: C5529G5JPQ) Alpha-Tocopherol (UNII: H4N855PNZ1) Urea (UNII: 8W8T17847W) Saccharomyces Cerevisiae (UNII: 978D8U419H) Arctium Lappa Root (UNII: 597E9BI3Z3) Trideceth-6 (UNII: 3T5PCR2H0C) Ruscus Aculeatus Root (UNII: ZW12V95I1Q) Betaine (UNII: 3SCV180C9W) Inositol (UNII: 4L6452S749) Taurine (UNII: 1EQV5MLY3D) Trehalose (UNII: B8WCK70T7I) Phytonadione (UNII: A034SE7857) Iodopropynyl Butylcarbamate (UNII: 603P14DHEB) Phenoxyethanol (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45156-5502-2 1 in 1 BOX 1 NDC:45156-5502-1 29 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 08/08/2011 Labeler - Skin Deep (833276566) Establishment Name Address ID/FEI Business Operations Skin Deep 833276566 manufacture