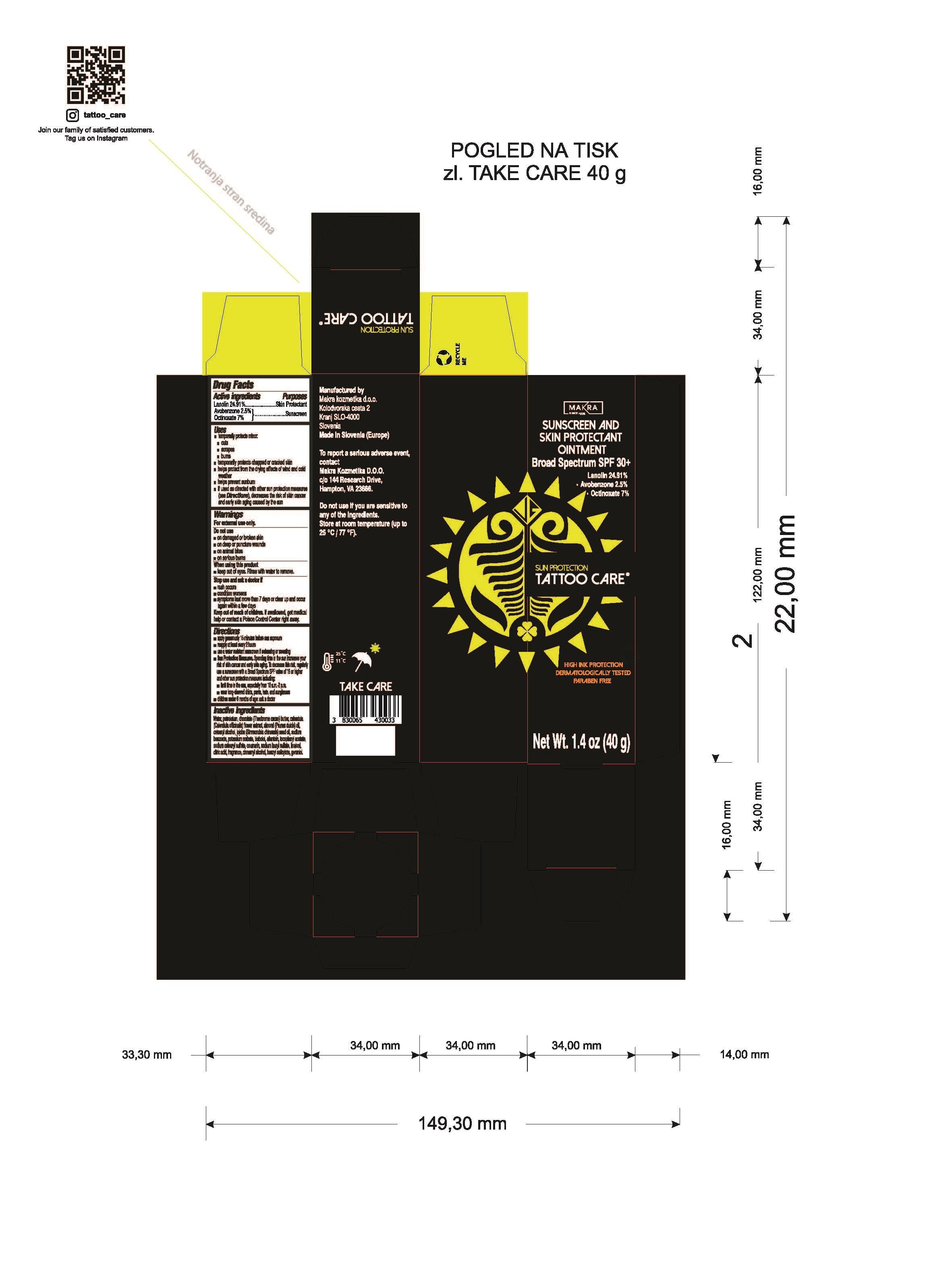
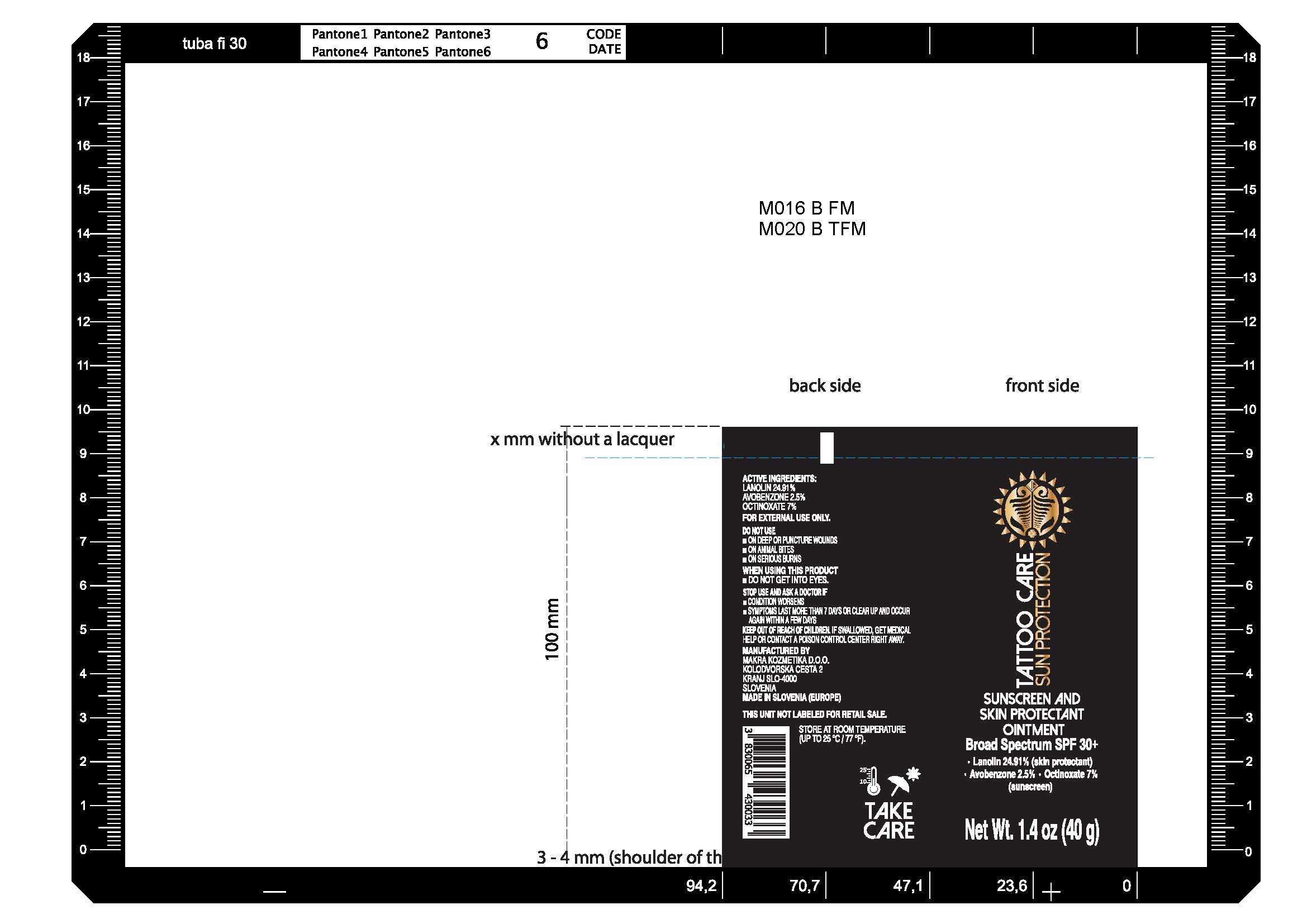
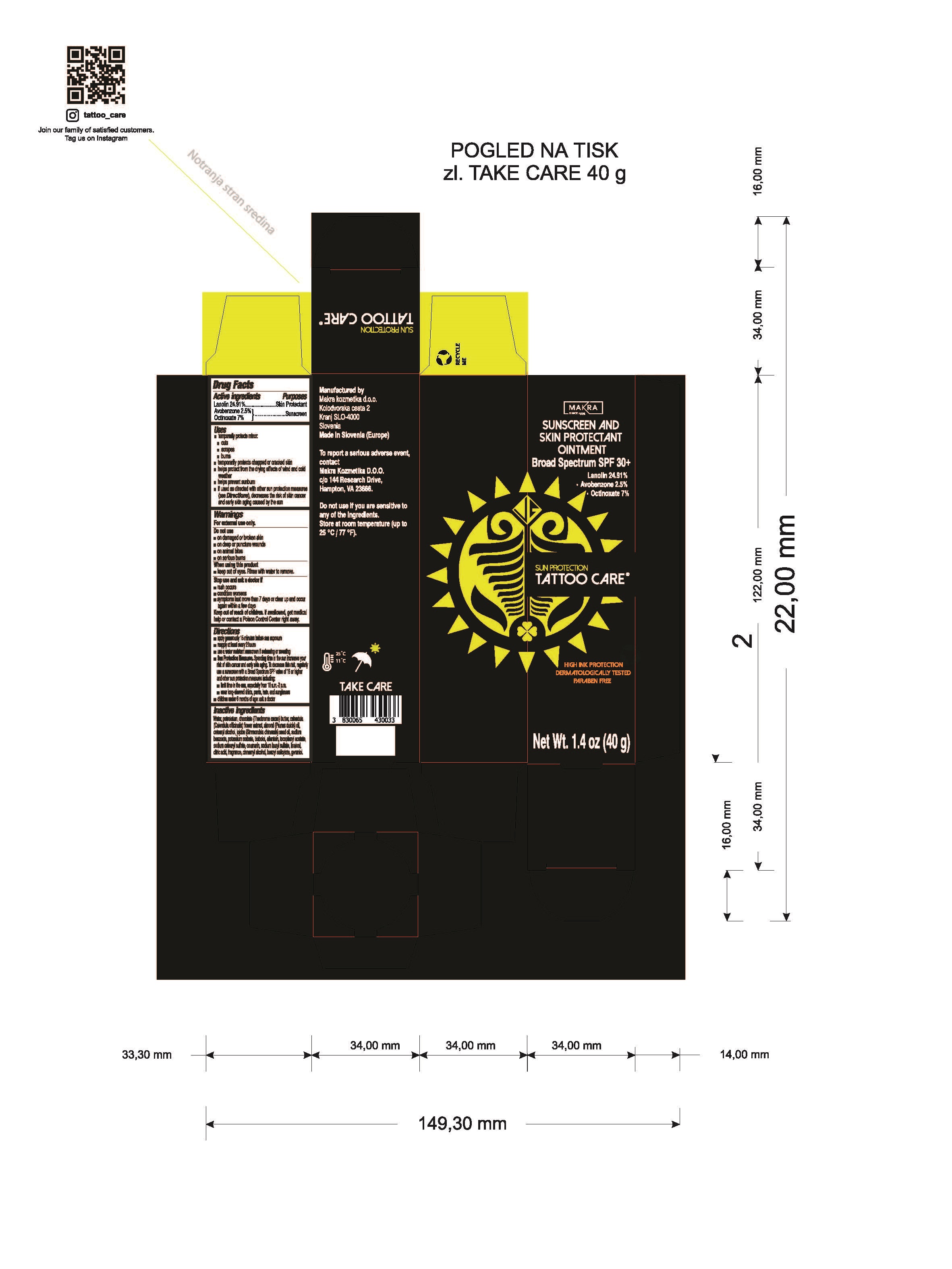
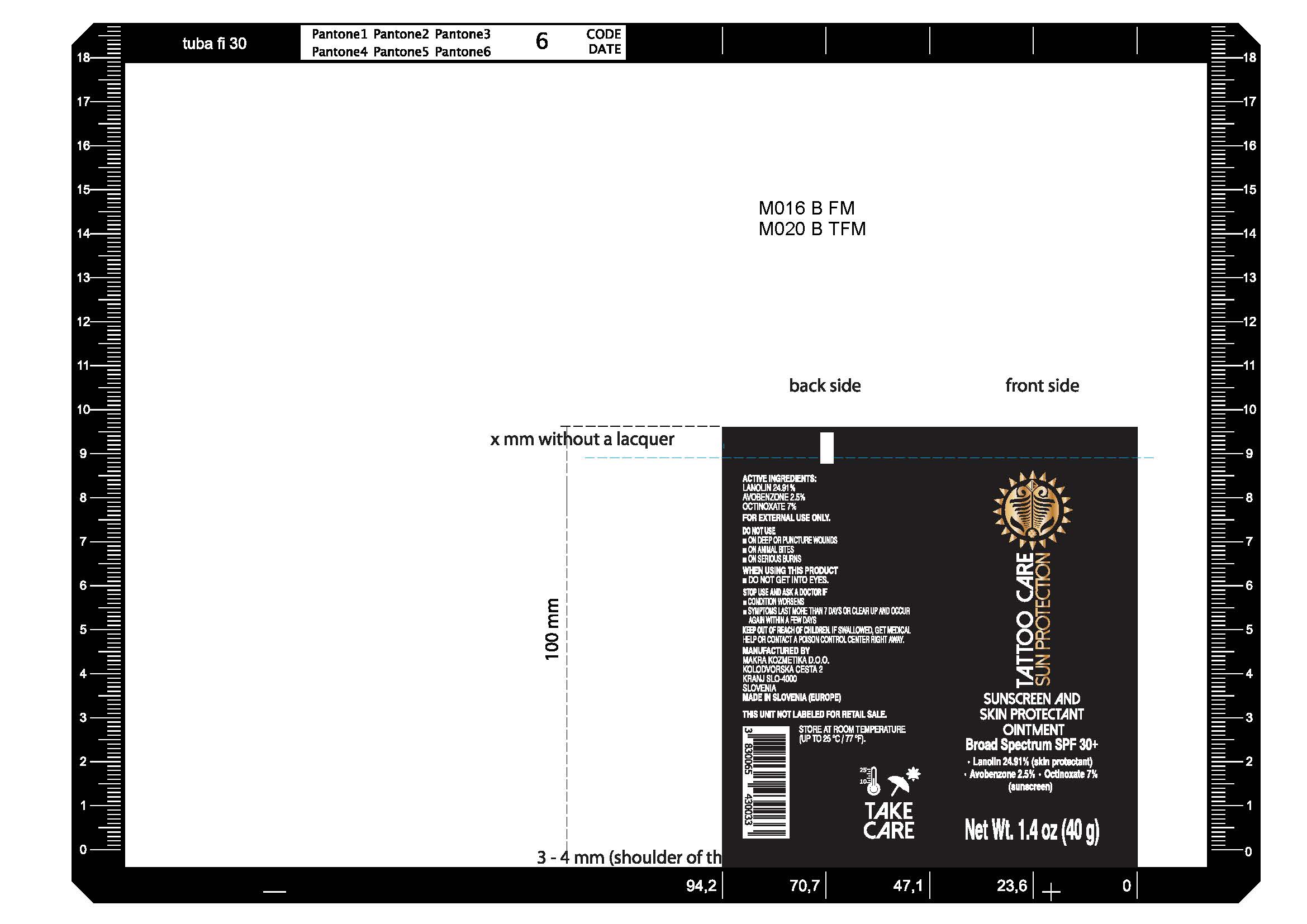
Label: SUN PROTECTION- lanolin, avobenzone, octinoxate ointment
- NDC Code(s): 82806-002-01
- Packager: MAKRA KOZMETIKA D.O.O.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purposes
-
Uses
- temporarily protects minor:
- temporarily protects chapped or cracked skin
- helps protect from the drying effects of wind and cold weather
- helps prevent sunburn
- if used as directed with other sun protection measures (see ), decreases the risk of skin cancer and early skin aging caused by the sun Directions
• cuts
• scrapes
• burns
- Warnings
-
Directions
- apply generously 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures.
- children under 6 months of age: ask a doctor
• limit time in the sun, especially from 10 a.m.-2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
-
Inactive ingredients
Water, petrolatum, chocolate (Theobroma cacao) butter, calendula (Calendula officinalis) flower extract, almond (Prunus dulcis) oil, cetearyl alcohol, jojoba (simmondsia chinesis) seed oil,sodium benzoate, potassium sorbate, bisbolol, allantoin, tocopheryl acetate, sodium cetearyl sulfate, coumarin, sodium lauryl sulfate, linalool, citric acid, fragrance, cinnamyl alchohol, benzyl salicylate, geraniol.
- Tattoo Care Sun Protection Sunscreen and Skin Protectant Ointment SPF 30+, 1.4 oz (40g) 82806-200-01
-
INGREDIENTS AND APPEARANCE
SUN PROTECTION
lanolin, avobenzone, octinoxate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82806-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANOLIN (UNII: 7EV65EAW6H) (LANOLIN - UNII:7EV65EAW6H) LANOLIN 249.1 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 25 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 70 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) COCOA BUTTER (UNII: 512OYT1CRR) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) ALMOND OIL (UNII: 66YXD4DKO9) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) JOJOBA OIL (UNII: 724GKU717M) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) LEVOMENOL (UNII: 24WE03BX2T) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SODIUM CETOSTEARYL SULFATE (UNII: 7ZBS06BH4B) COUMARIN (UNII: A4VZ22K1WT) SODIUM LAURYL SULFATE (UNII: 368GB5141J) LINALOOL, (+/-)- (UNII: D81QY6I88E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CINNAMYL ALCOHOL (UNII: SS8YOP444F) BENZYL SALICYLATE (UNII: WAO5MNK9TU) GERANIOL (UNII: L837108USY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82806-002-01 1 in 1 CARTON 09/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 09/01/2022 Labeler - MAKRA KOZMETIKA D.O.O. (537064093) Registrant - MAKRA KOZMETIKA D.O.O. (537064093) Establishment Name Address ID/FEI Business Operations MAKRA KOZMETIKA D.O.O. 537064093 manufacture(82806-002)