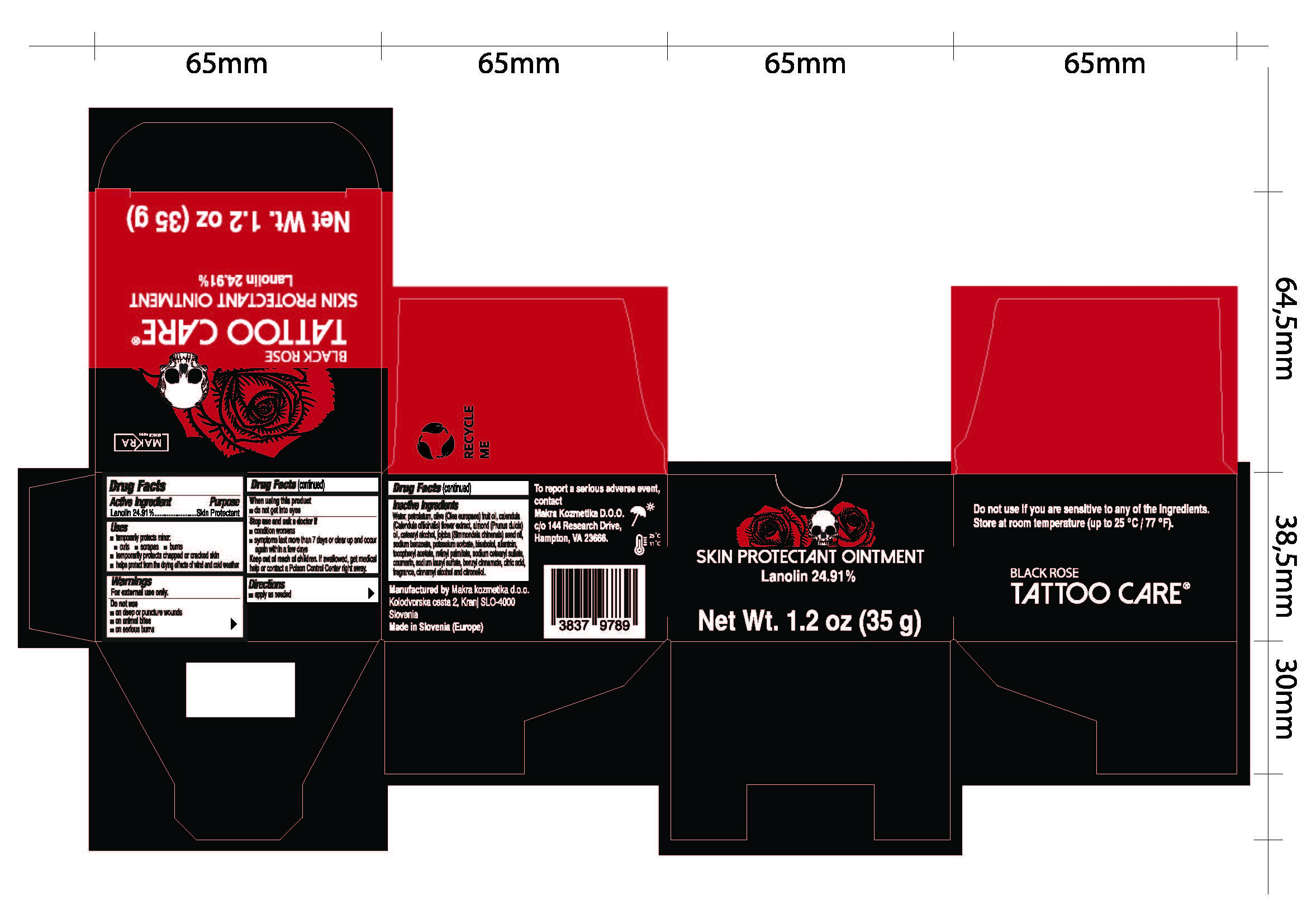
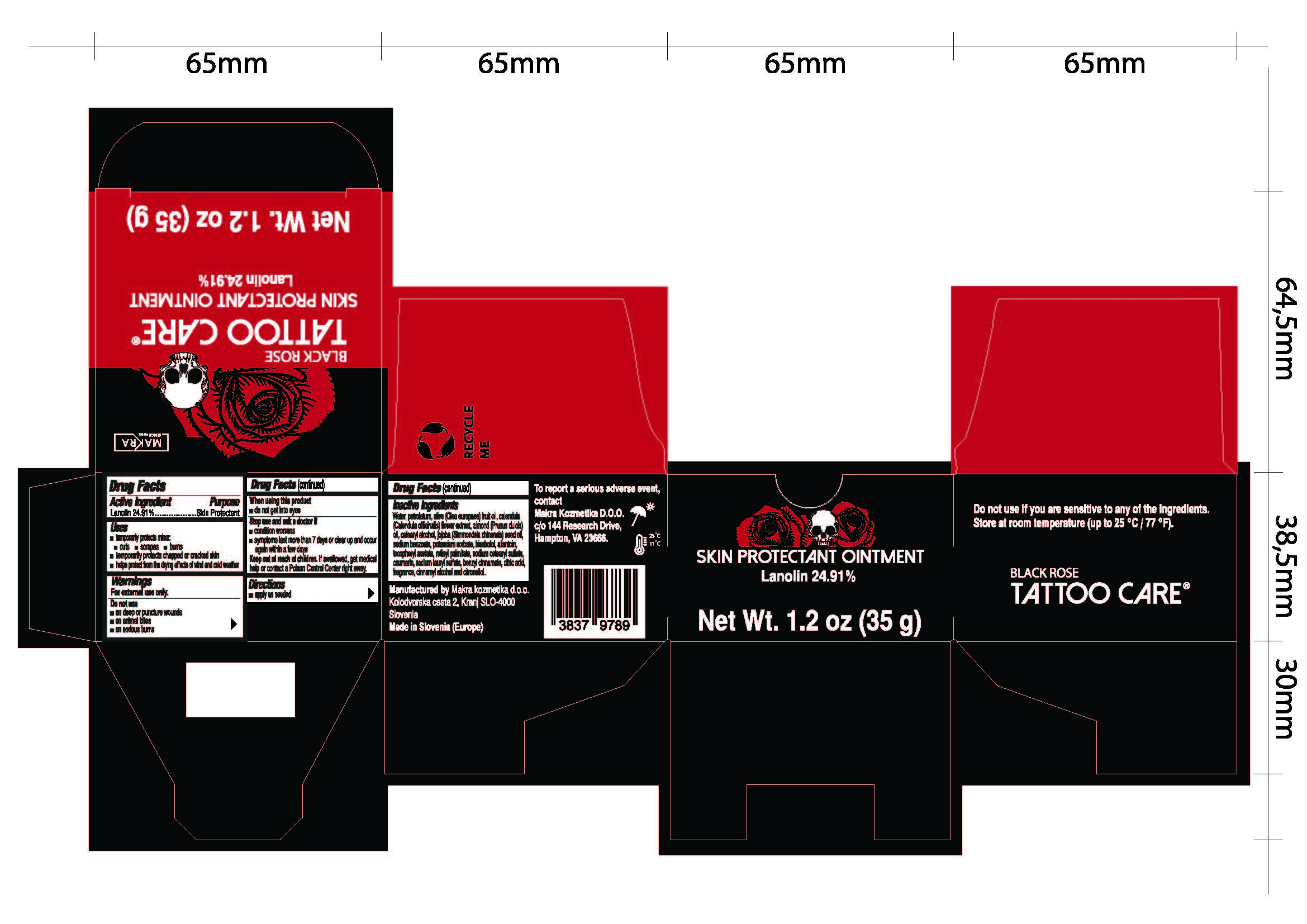
Label: BLACK ROSE- lanolin ointment
- NDC Code(s): 82806-001-01
- Packager: MAKRA KOZMETIKA D.O.O.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- When using this product
- Stop use and ask a doctor if
- Directions
-
Inactive ingredients
Water, petrolatum, olive (Olea europea) fruit oil, calendula (Calendula officinalis) flower extract, almond (Prunus dulcis) oil, cetearyl alcohol, jojoba (Simmondsia chinesis) seed oil, sodium benzoate, potassium sorbate, bisabolol, allantoin, tocopheryl acetate, retinyl palmitate, sodium cetearyl sulfate, coumarin, sodium lauryl sulfate, benzyl cinnamate, citric acid, fragrance, cinnamyl alcohol and citronellol.
- Tattoo Care Black Rose Skin Protectant Ointment, 1.2 oz (35g) 82806-100-01
-
INGREDIENTS AND APPEARANCE
BLACK ROSE
lanolin ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82806-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANOLIN (UNII: 7EV65EAW6H) (LANOLIN - UNII:7EV65EAW6H) LANOLIN 249.1 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) OLIVE OIL (UNII: 6UYK2W1W1E) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) ALMOND OIL (UNII: 66YXD4DKO9) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) JOJOBA OIL (UNII: 724GKU717M) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SODIUM CETOSTEARYL SULFATE (UNII: 7ZBS06BH4B) COUMARIN (UNII: A4VZ22K1WT) SODIUM LAURYL SULFATE (UNII: 368GB5141J) BENZYL CINNAMATE (UNII: V67O3RO97U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CINNAMYL ALCOHOL (UNII: SS8YOP444F) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82806-001-01 1 in 1 CARTON 09/01/2022 1 35 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 09/01/2022 Labeler - MAKRA KOZMETIKA D.O.O. (537064093) Registrant - MAKRA KOZMETIKA D.O.O. (537064093) Establishment Name Address ID/FEI Business Operations MAKRA KOZMETIKA D.O.O. 537064093 manufacture(82806-001)