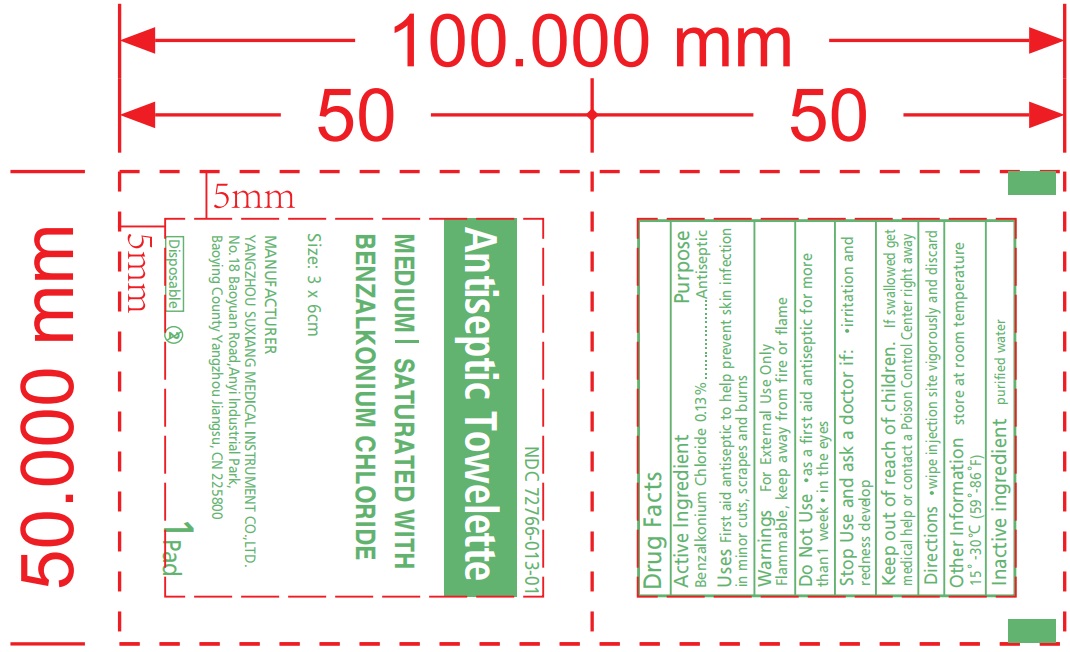
Label: BENZALKONIUM CHLORIDE ANTISEPTIC TOWELETTE- benzalkonium chloride towelette swab
- NDC Code(s): 72766-013-01
- Packager: Yangzhou Suxiang Medical Instrument Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Uses
- Warnings
- Directions
- Inactive Ingredients
- Antiseptic Towelette
-
INGREDIENTS AND APPEARANCE
BENZALKONIUM CHLORIDE ANTISEPTIC TOWELETTE
benzalkonium chloride towelette swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72766-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 8 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72766-013-01 1 in 1 PACKET; Type 0: Not a Combination Product 08/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 08/31/2022 Labeler - Yangzhou Suxiang Medical Instrument Co., Ltd. (543387280)