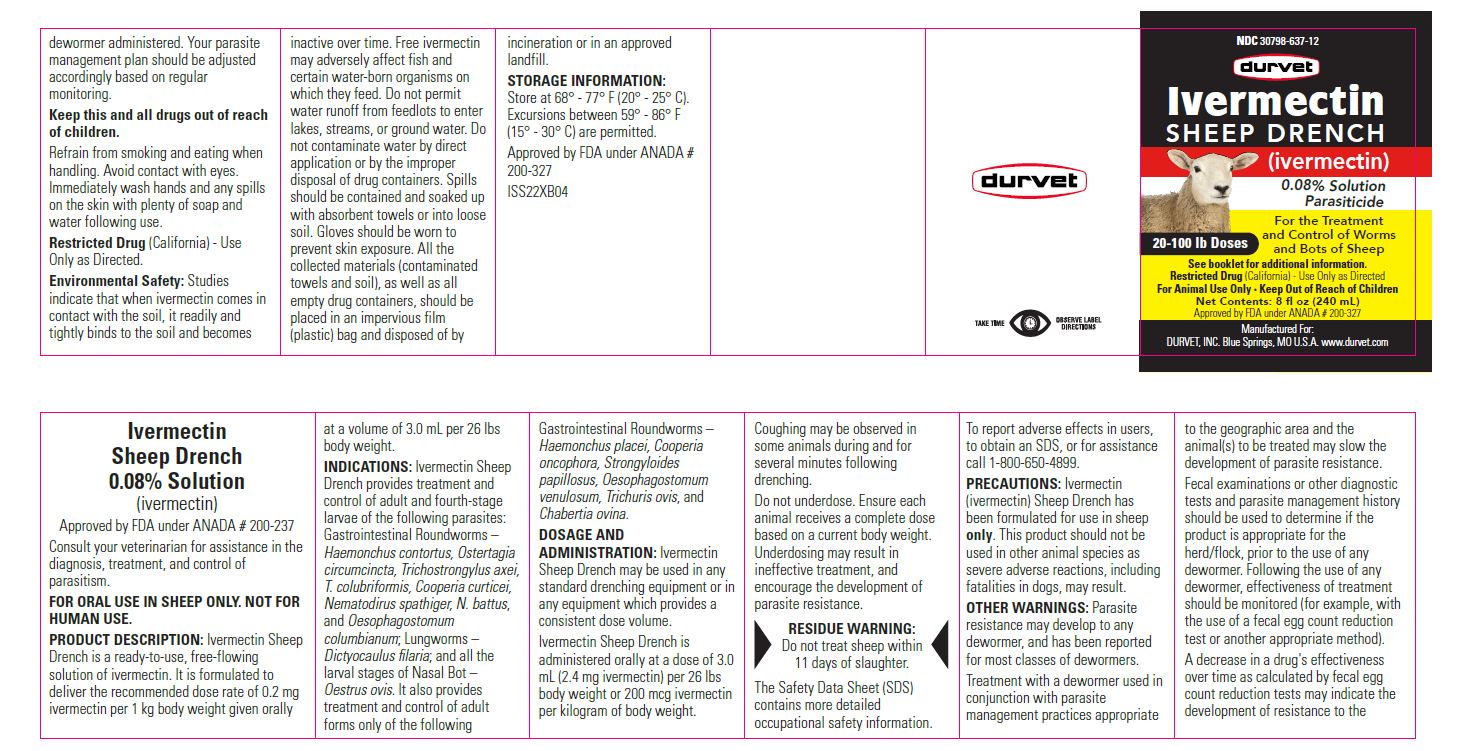
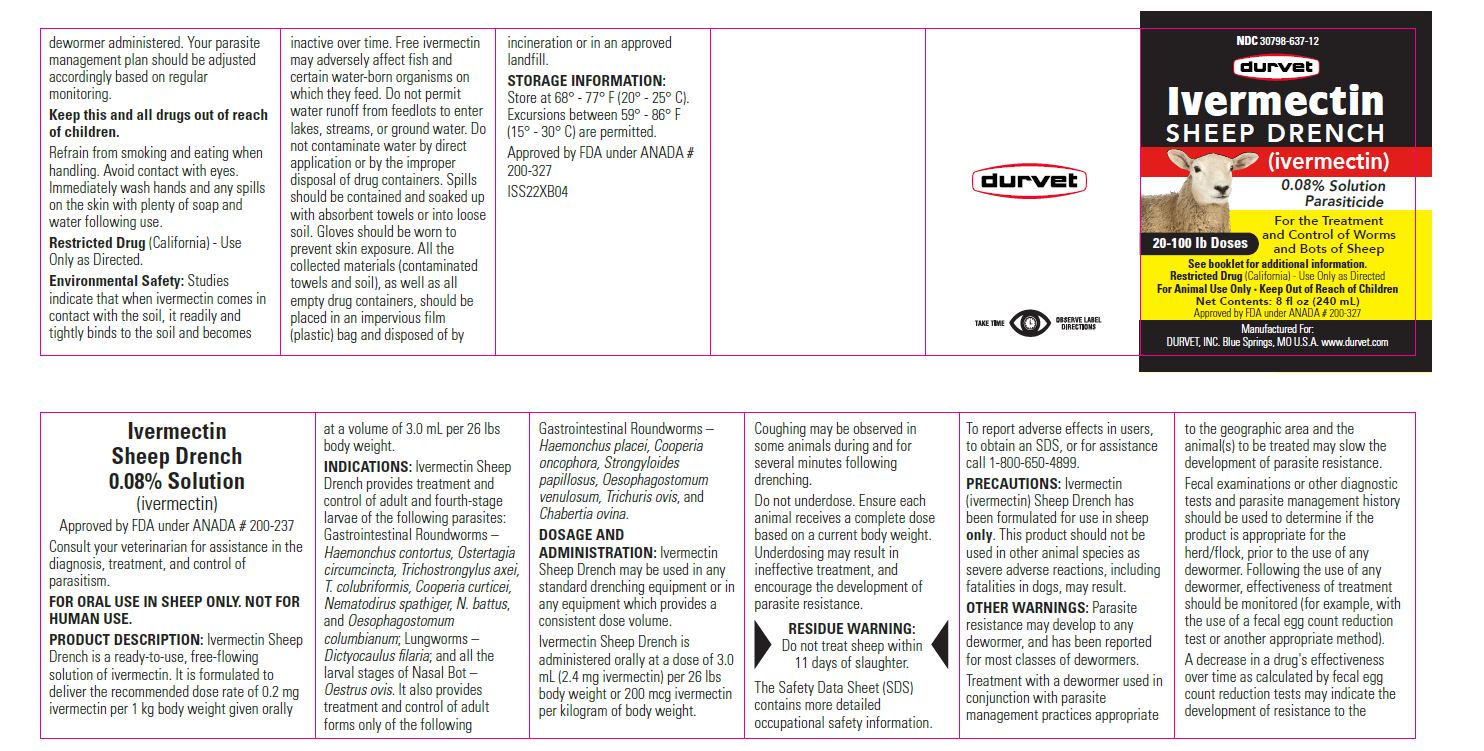
Label: IVERMECTIN SHEEP DRENCH- ivermectin solution
- NDC Code(s): 30798-637-12, 30798-637-16
- Packager: Durvet
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated August 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INDICATIONS & USAGE
For the Treatment and Control of Worms and Bots of Sheep
- 20- 100 lb Doses (240 mL)
- 83- 100 lb Doses (960 mL)
See booklet for additional information.
Restricted Drug (California) - Use Only as Directed
For Animal Use Only • Keep Out of Reach of Children
Approved by FDA under ANADA # 200-327
Manufactured For:
DURVET, INC. Blue Springs, MO U.S.A. www.durvet.com - INFORMATION FOR OWNERS/CAREGIVERS
- PRODUCT DESCRIPTION
-
INDICATIONS:
Ivermectin Sheep Drench provides treatment and control of adult and fourth-stage larvae of the following parasites: Gastrointestinal Roundworms – Haemonchus contortus, Ostertagia circumcincta, Trichostrongylus axei, T. colubriformis, Cooperia curticei, Nematodirus spathiger, N. battus, and Oesophagostomum columbianum; Lungworms – Dictyocaulus filaria; and all the larval stages of Nasal Bot – Oestrus ovis. It also provides treatment and control of adult forms only of the following Gastrointestinal Roundworms – Haemonchus placei, Cooperia oncophora, Strongyloides papillosus, Oesophagostomum venulosum, Trichuris ovis, and Chabertia ovina.
-
DOSAGE AND ADMINISTRATION:
Ivermectin Sheep Drench may be used in any standard drenching equipment or in any equipment which provides a consistent dose volume.
Ivermectin Sheep Drench is administered orally at a dose of 3.0 mL (2.4 mg ivermectin) per 26 lbs body weight or 200 mcg ivermectin per kilogram of body weight.
Coughing may be observed in some animals during and for several minutes following drenching.
Do not underdose. Ensure each animal receives a complete dose based on a current body weight. Underdosing may result in ineffective treatment, and encourage the development of parasite resistance. - RESIDUE WARNING:
- ADVERSE REACTIONS
- PRECAUTIONS:
-
OTHER WARNINGS:
Parasite resistance may develop to any dewormer, and has been reported for most classes of dewormers.
Treatment with a dewormer used in conjunction with parasite management practices appropriate to the geographic area and the animal(s) to be treated may slow the development of parasite resistance.
Fecal examinations or other diagnostic tests and parasite management history should be used to determine if the product is appropriate for the herd/flock, prior to the use of any dewormer. Following the use of any dewormer, effectiveness of treatment should be monitored (for example, with the use of a fecal egg count reduction test or another appropriate method).
A decrease in a drug's effectiveness over time as calculated by fecal egg count reduction tests may indicate the development of resistance to the dewormer administered. Your parasite management plan should be adjusted accordingly based on regular monitoring. - OTHER SAFETY INFORMATION
-
Environmental Safety:
Studies indicate that when ivermectin comes in contact with the soil, it readily and tightly binds to the soil and becomes inactive over time. Free ivermectin may adversely affect fish and certain water-born organisms on which they feed. Do not permit water runoff from feedlots to enter lakes, streams, or ground water. Do not contaminate water by direct application or by the improper disposal of drug containers. Spills should be contained and soaked up with absorbent towels or into loose soil. Gloves should be worn to prevent skin exposure. All the collected materials (contaminated towels and soil), as well as all empty drug containers, should be placed in an impervious film (plastic) bag and disposed of by incineration or in an approved landfill.
- STORAGE INFORMATION:
- HOW SUPPLIED
- 240 mL (ISS22XB04)
- 960 mL (ISS20XB09))
-
INGREDIENTS AND APPEARANCE
IVERMECTIN SHEEP DRENCH
ivermectin solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:30798-637 Route of Administration Oral Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IVERMECTIN (UNII: 8883YP2R6D) (IVERMECTIN - UNII:8883YP2R6D) IVERMECTIN 0.8 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30798-637-12 6 in 1 CASE 1 240 mL in 1 JUG 2 NDC:30798-637-16 4 in 1 CASE 2 960 mL in 1 JUG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200327 01/30/2009 Labeler - Durvet (056387798) Establishment Name Address ID/FEI Business Operations First Priority Incorporated 179925722 manufacture