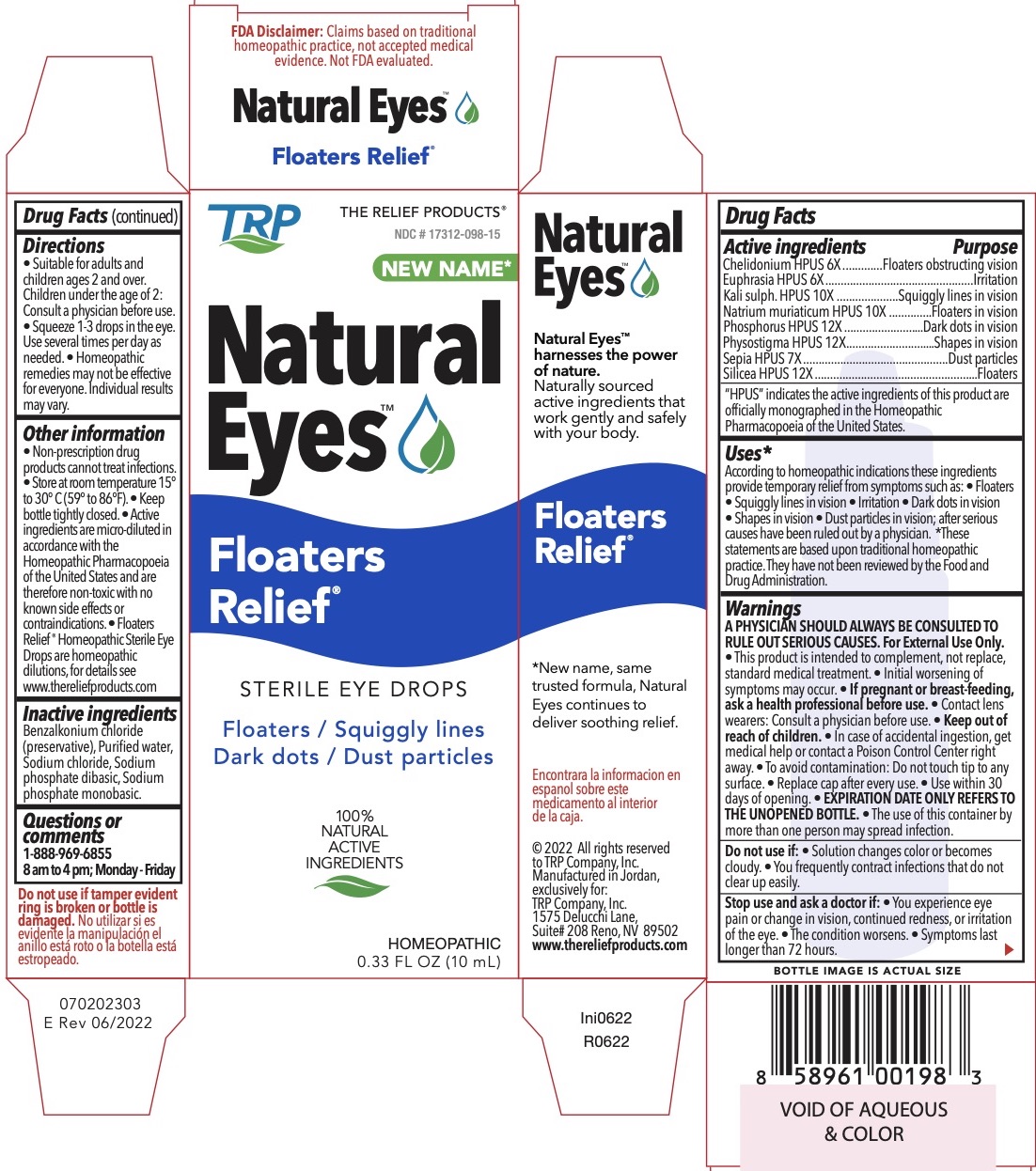
Label: FLOATERS RELIEF- chelidonium majus, physostigma venenosum seed, euphrasia stricta,potassium sulfate, sodium chloride, phosphorus, silicon dioxide, sepia officinalis juice liquid
- NDC Code(s): 17312-098-15
- Packager: TRP Company
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 9, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients Purpose Chelidonium HPUS 6x Floaters obstructing vision Physostigma HPUS 12x Shapes in vision Euphrasia HPUS 6x Irritation Kali Sulph. HPUS 10x Squiggly lines in vision Natrium muriaticum HPUS 10x Floaters in vision Phosphorus HPUS 12x Dark dots in vision Silicea HPUS 12x Blurry vision Sepia HPUS 7x Dust particles "HPUS" indicates the active ingredients are in the Homeopathic Pharmacopoeia of the United States.
- PURPOSE
-
INDICATIONS & USAGE
Uses:*
According to homeopathic indications these ingredients provide temporary relief from symptoms such as:• Floaters • Squiggly lines in vision • Irritation • Dark dots in vision • Shapes in vision • Dust particles in vision; after serious causes have been ruled out by a physician.
*These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- WARNINGS
- Do not use:
- Stop use and ask a doctor if:
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
SPL UNCLASSIFIED SECTION
Other information:
- Non-prescription drug products cannot treat infections.
- Store at room temperature 15° to 30° C (59° to 86°F). • Keep bottle tightly closed. • Active ingredients are micro-diluted in accordance with the Homeopathic Pharmacopoeia of the United States and are therefore non-toxic with no known side effects or contraindications. • Eye Floaters ® Homeopathic Sterile Eye Drops are homeopathic dilutions, for details see www.thereliefproducts.com.
- INACTIVE INGREDIENT
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLOATERS RELIEF
chelidonium majus, physostigma venenosum seed, euphrasia stricta,potassium sulfate, sodium chloride, phosphorus, silicon dioxide, sepia officinalis juice liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17312-098 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 1 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 7 [hp_X] in 1 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 6 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 12 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 10 [hp_X] in 1 mL POTASSIUM SULFATE (UNII: 1K573LC5TV) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM SULFATE 10 [hp_X] in 1 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 6 [hp_X] in 1 mL PHYSOSTIGMA VENENOSUM SEED (UNII: CJV9E9IIOA) (PHYSOSTIGMA VENENOSUM SEED - UNII:CJV9E9IIOA) PHYSOSTIGMA VENENOSUM SEED 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17312-098-15 1 in 1 PACKAGE 08/09/2022 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/09/2022 Labeler - TRP Company (105185719)