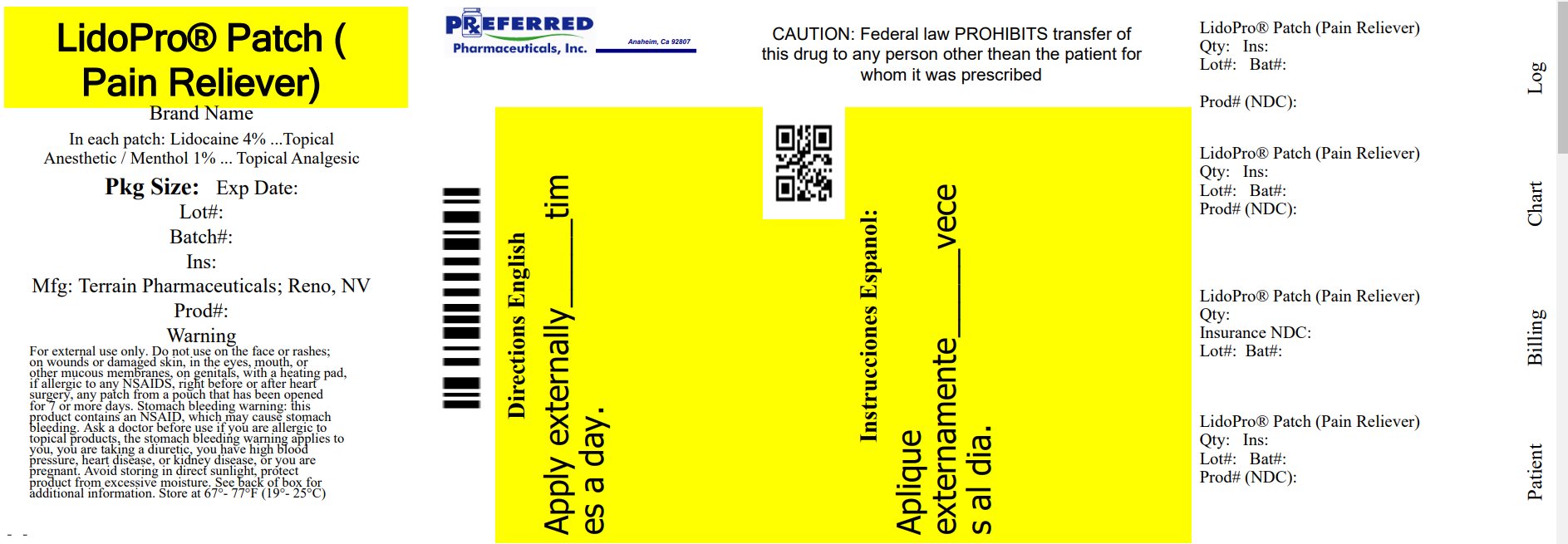
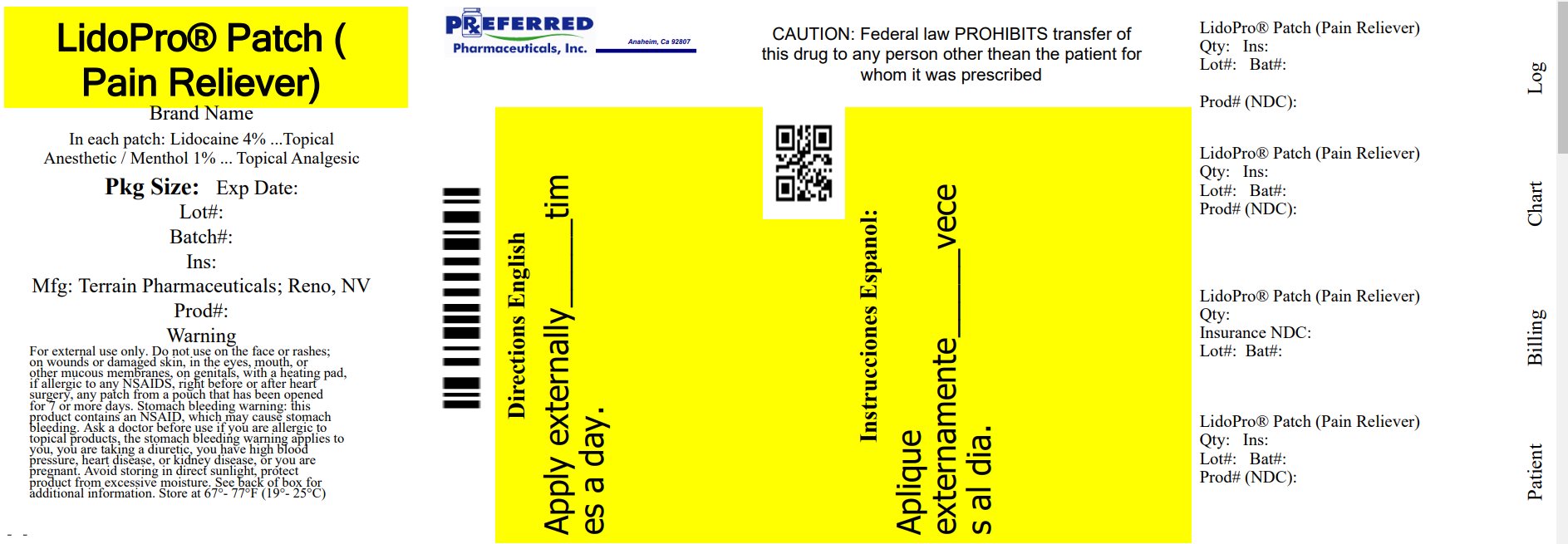
Label: LIDOPRO PATCH- lidocaine and menthol patch
- NDC Code(s): 68788-8630-1
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 53225-1123
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
-
Active IngredientLidocaine 4%
-
Purpose Topical Anesthetic
-
Active IngredientMenthol 1%
-
Purpose Topical Analgesic
-
UsesFor the temporary relief of pain.
-
Warnings For external use only - Do not use - • on the face or rashes, on wounds or damaged skin - • in the eyes, mouth, or other mucous membranes - • on genitals - • with a heating pad - • right before or after ...
-
DirectionsAdults 18 years and older: • clean and dry affected area - • open pouch and remove one patch - • remove protective film from patch - • apply one patch to the affected area of pain and leave in place ...
-
Other information• some individuals may not experience pain relief until several minutes or hours after applying the patch - • avoid storing product in direct sunlight - • protect product from excessive moisture - ...
-
Inactive Ingredientsdihydroxyaluminum aminoacetate, glycerol, methylparaben, polysorbate 80, propylene glycol, sodium polyacrylate, tartaric acid, water
-
Questions?877-985-8377 - Relabeled By: Preferred Pharmaceuticals Inc.
-
Principal Display Panel
-
INGREDIENTS AND APPEARANCEProduct Information