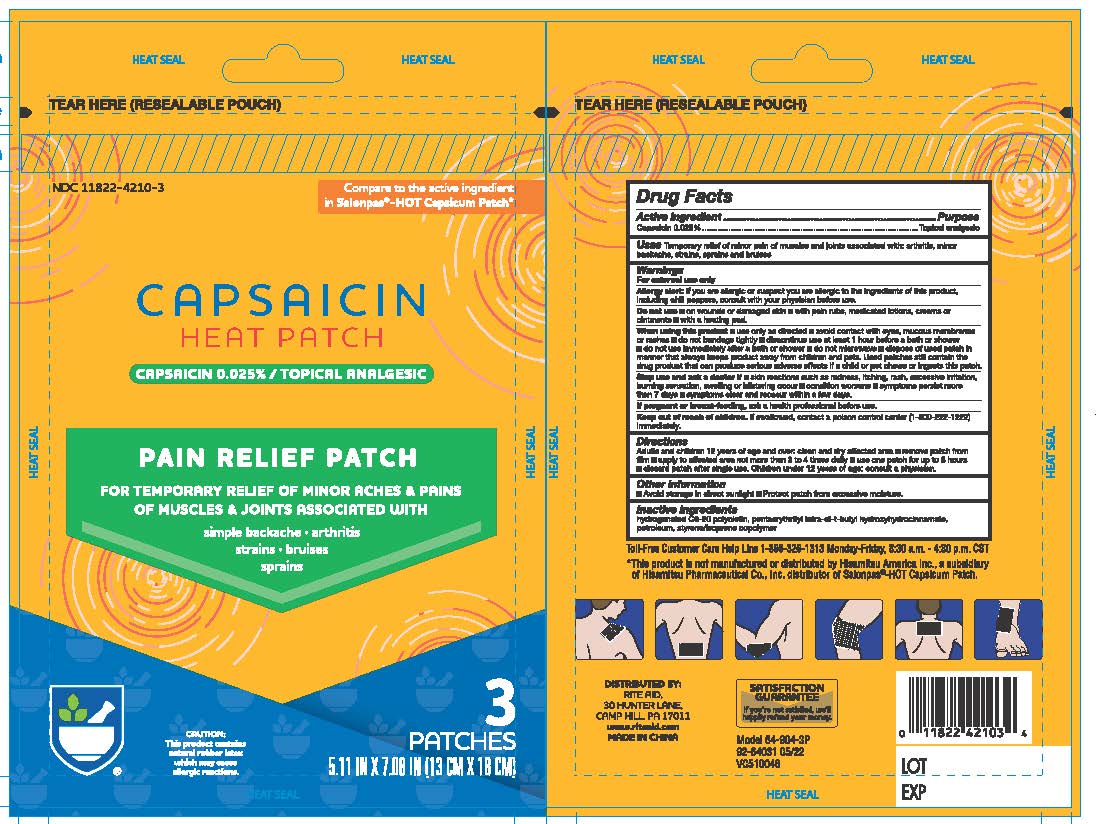
Label: CAPSAICIN HEAT PATCHES PAIN RELIEF- capsaicin patch
- NDC Code(s): 11822-4210-3
- Packager: Rite Aid
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
For External Use Only
Allergy alert
If you are allergic or suspect you are allergic to the ingredients of this product, including chill peppers, consult with your physician before use.
Do Not Use:
- On wounds or damaged skin
- with pain rubs, medicated lotions, creams, or ointments
- with heating pads
When Using this product
- Use only as directed
- Avoid contact with eyes, mucous membranes or rashes
- do not bandage tightly
- discontinue use at least 1 hour before a bath or shower
- do not use immediately after a bath or shower
- do not microwave
- dispose of used patch in a manner that always keeps products away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingest this patch.
- Inactive Ingredients:
- Directions:
- Other Information
- Inactive Ingredients
- QUESTIONS
- LABEL
-
INGREDIENTS AND APPEARANCE
CAPSAICIN HEAT PATCHES PAIN RELIEF
capsaicin patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-4210 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.025 g in 100 g Inactive Ingredients Ingredient Name Strength PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) LIQUID PETROLEUM (UNII: 6ZAE7X688J) STYRENE/ISOPRENE/STYRENE BLOCK COPOLYMER (UNII: K7S96QM8DV) HYDROGENATED C6-20 POLYOLEFIN (100 CST) (UNII: 39EYQ1W9RB) Product Characteristics Color Score Shape RECTANGLE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-4210-3 3 in 1 BOX 08/01/2022 1 9 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/01/2022 Labeler - Rite Aid (014578892) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co.,Ltd. 529128763 manufacture(11822-4210)