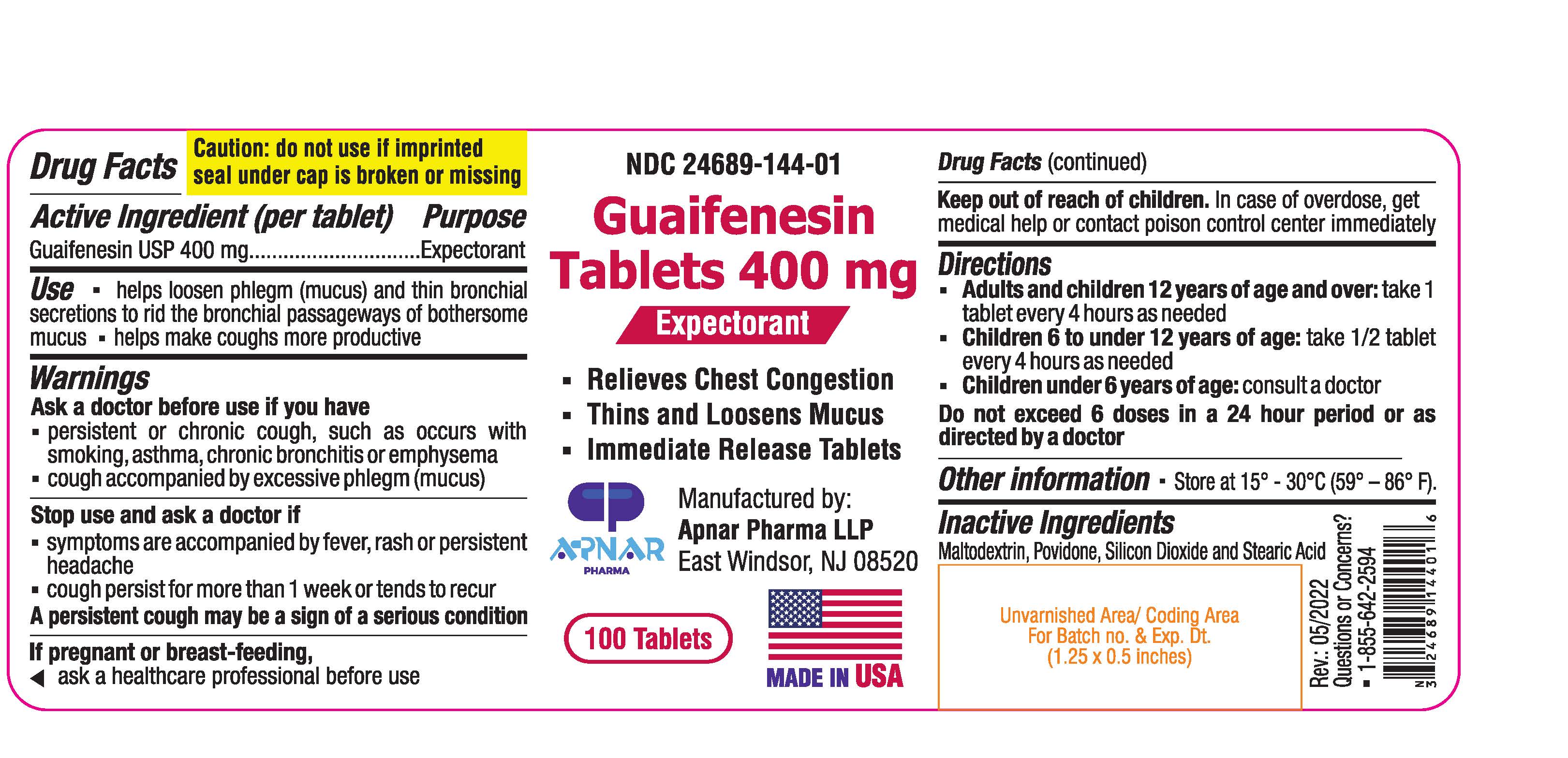
Label: GUAIFENESIN tablet
- NDC Code(s): 24689-144-01
- Packager: APNAR PHARMA LP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- BOXED WARNING (What is this?)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Ask doctor before use if you have
- persistent or chronic cough, such as occurs with smoking, asthma, chronic bronchitis or emphysema
- cough accompanied by excessive phlegm (mucus)
Stop use and ask a doctor if
- Symptoms are accompanied by fever, rash or persistent headache
- cough persists for more than 1 week or tends to recur
A persistent cough may be a sign of a serious condition
If pregnant or breast-feeding, ask a healthcare professional before use.
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN
guaifenesin tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24689-144 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg Inactive Ingredients Ingredient Name Strength MALTODEXTRIN (UNII: 7CVR7L4A2D) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white Score 2 pieces Shape ROUND (Round shaped standard concave) Size 10mm Flavor Imprint Code A;G1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24689-144-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/05/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/05/2022 Labeler - APNAR PHARMA LP (079568229) Establishment Name Address ID/FEI Business Operations INVAHEALTH INC 116840615 label(24689-144) , pack(24689-144) Establishment Name Address ID/FEI Business Operations APNAR PHARMA LLP 118530917 manufacture(24689-144) , analysis(24689-144) , pack(24689-144)