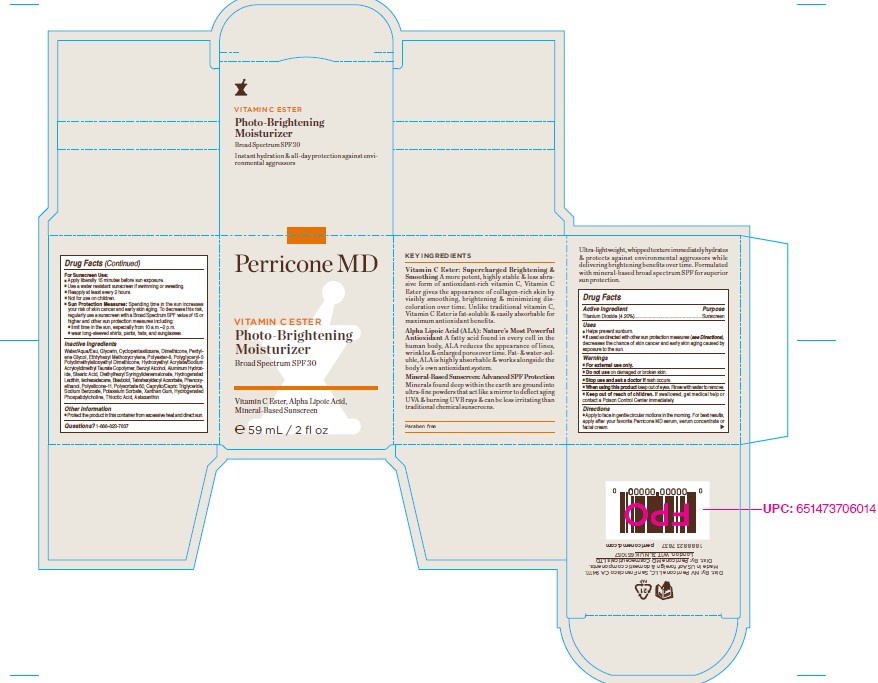
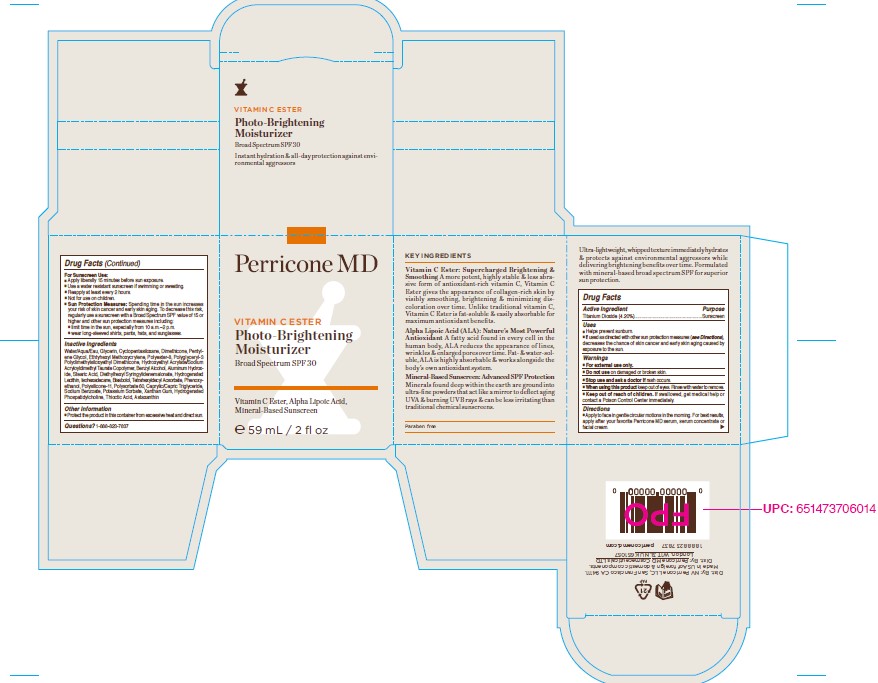
Label: VITAMIN C ESTER PHOTO-BRIGHTENING MOISTURIZER BROAD SPECTRUM SPF 30 59ML- titanium dioxide cream
- NDC Code(s): 45634-254-20
- Packager: N.V Perricone LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 10, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PURPOSE
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- USER SAFETY WARNINGS
- WARNINGS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITAMIN C ESTER PHOTO-BRIGHTENING MOISTURIZER BROAD SPECTRUM SPF 30 59ML
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45634-254 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.48 g in 59 g Inactive Ingredients Ingredient Name Strength LEVOMENOL (UNII: 24WE03BX2T) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 60 (UNII: CAL22UVI4M) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) XANTHAN GUM (UNII: TTV12P4NEE) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PENTYLENE GLYCOL (UNII: 50C1307PZG) BENZYL ALCOHOL (UNII: LKG8494WBH) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ISOHEXADECANE (UNII: 918X1OUF1E) THIOCTIC ACID (UNII: 73Y7P0K73Y) PHOSPHOLIPASE D1 (UNII: RO5LD48GLV) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HAEMATOCOCCUS PLUVIALIS (UNII: 31T0FF0472) TOCOPHEROL (UNII: R0ZB2556P8) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE (4000 MPA.S) (UNII: RLA2U05Z4Q) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45634-254-20 82 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 07/28/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 07/28/2022 Labeler - N.V Perricone LLC (054414243) Establishment Name Address ID/FEI Business Operations Dimensional Merchandising Inc (DMI) 076693183 manufacture(45634-254)