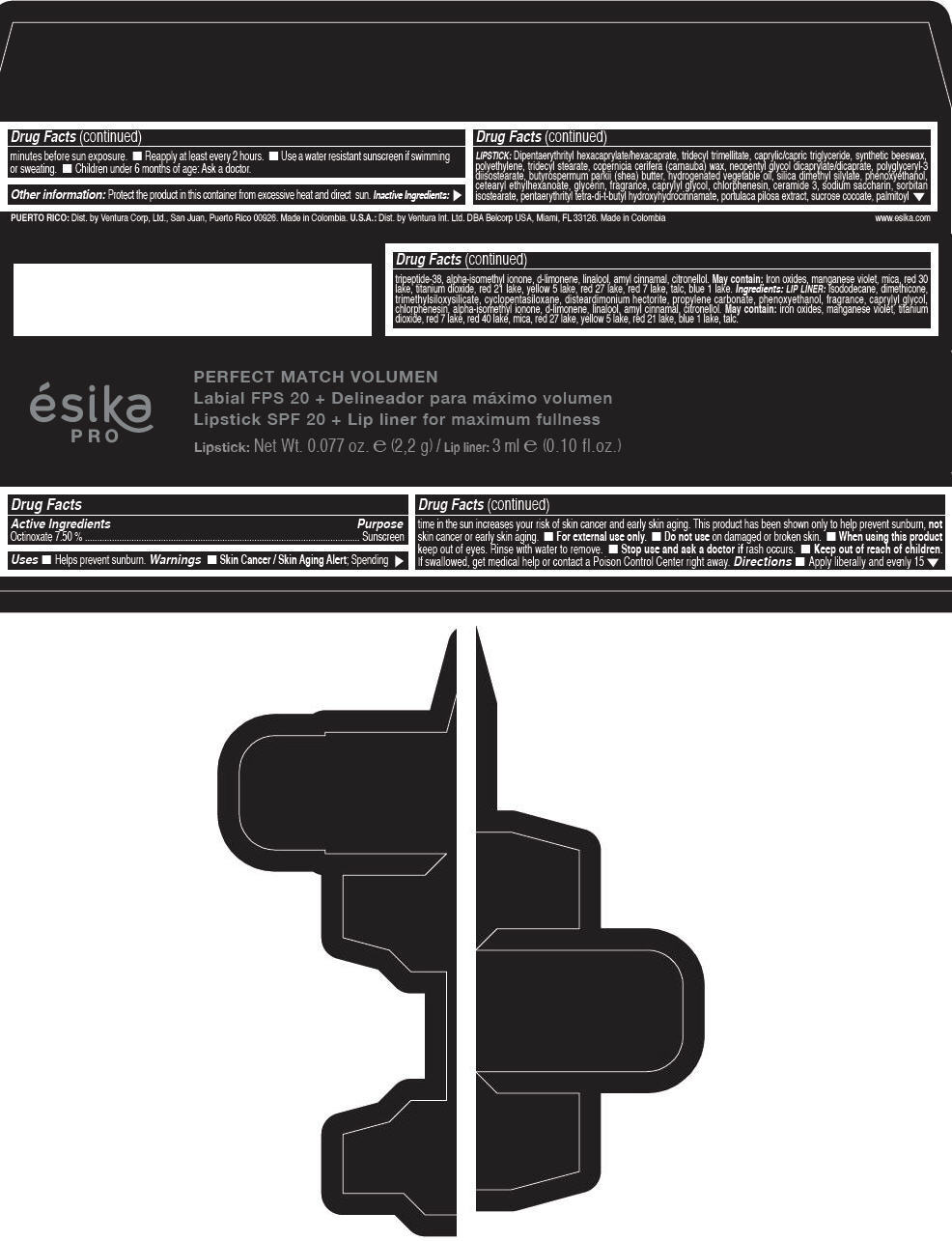
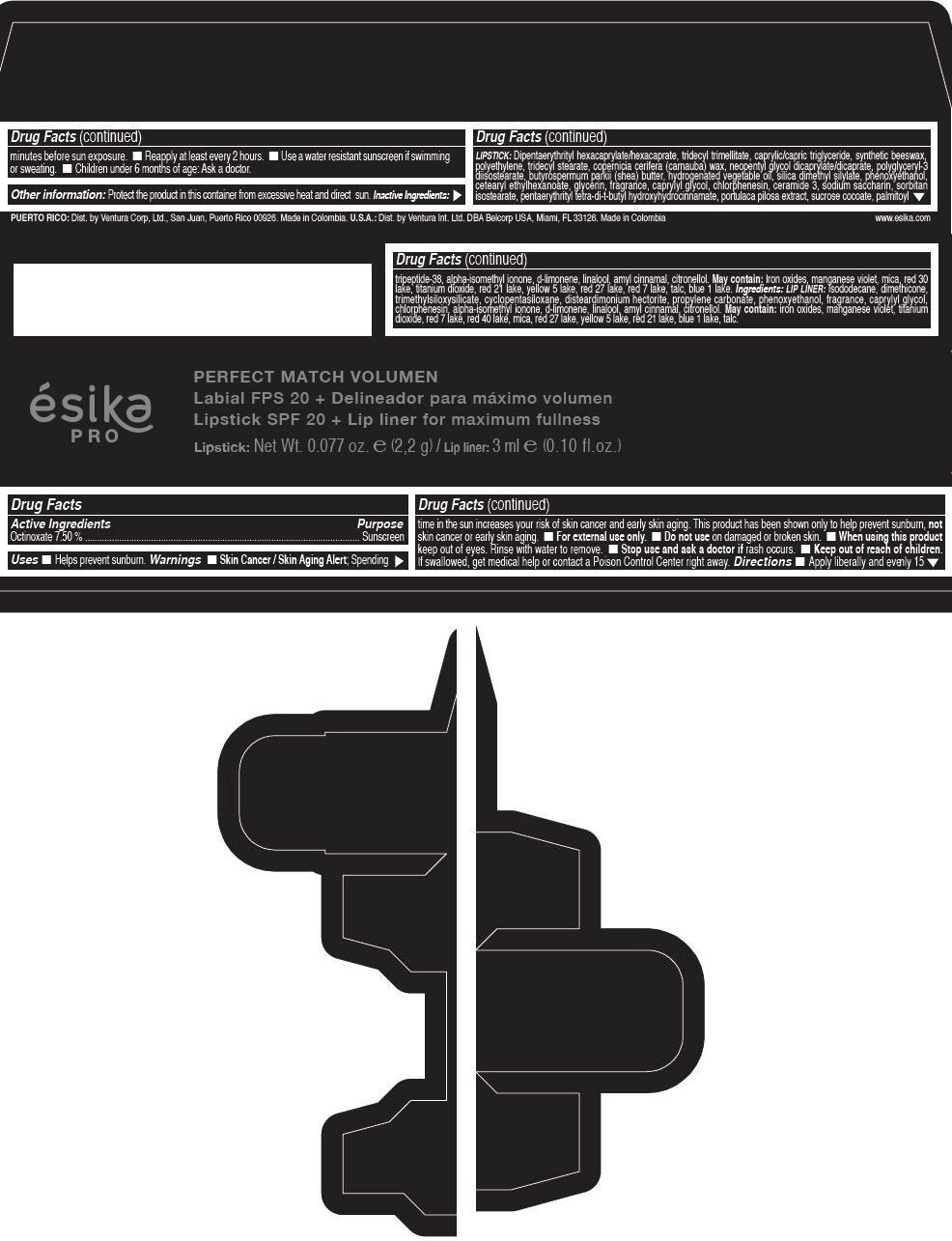
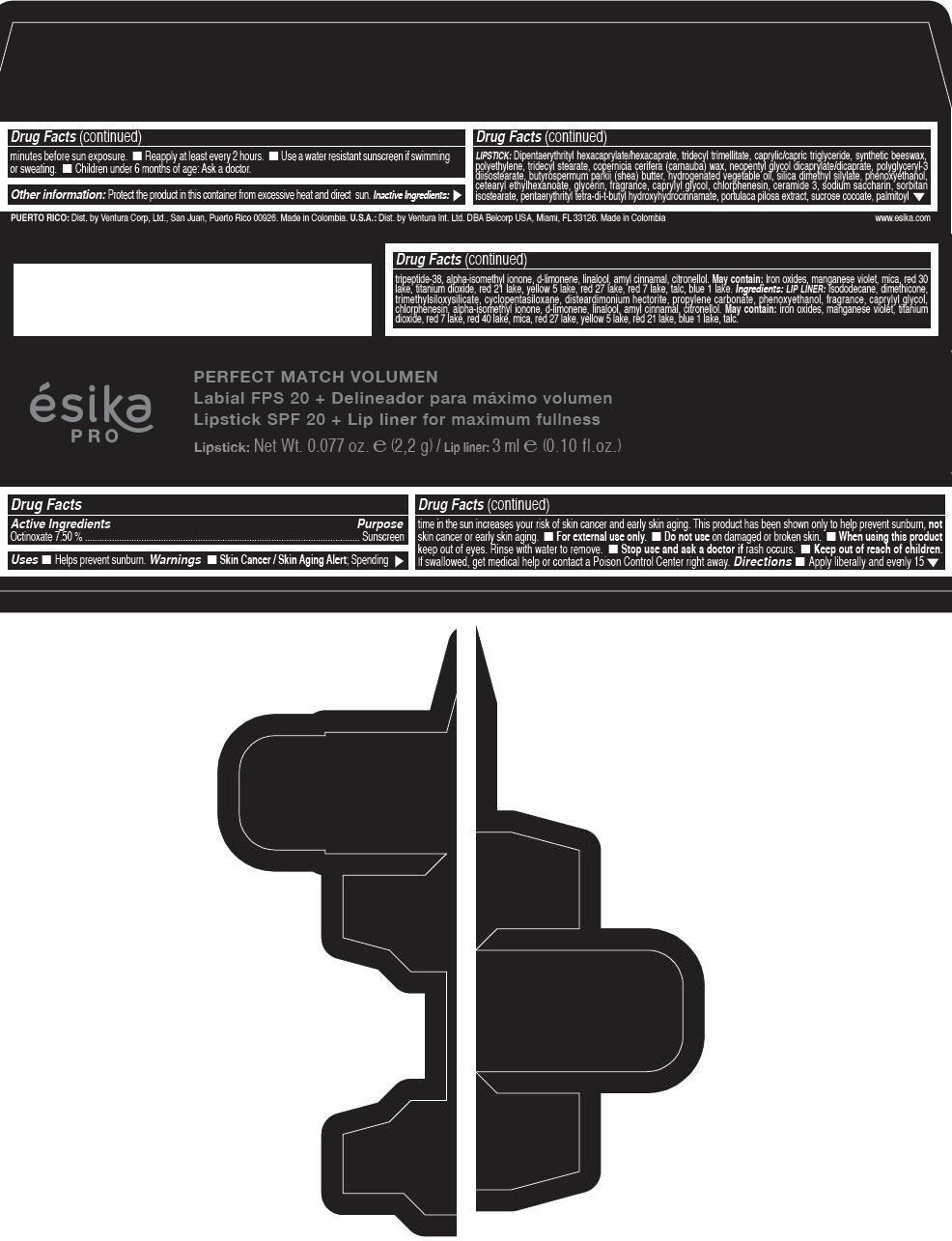
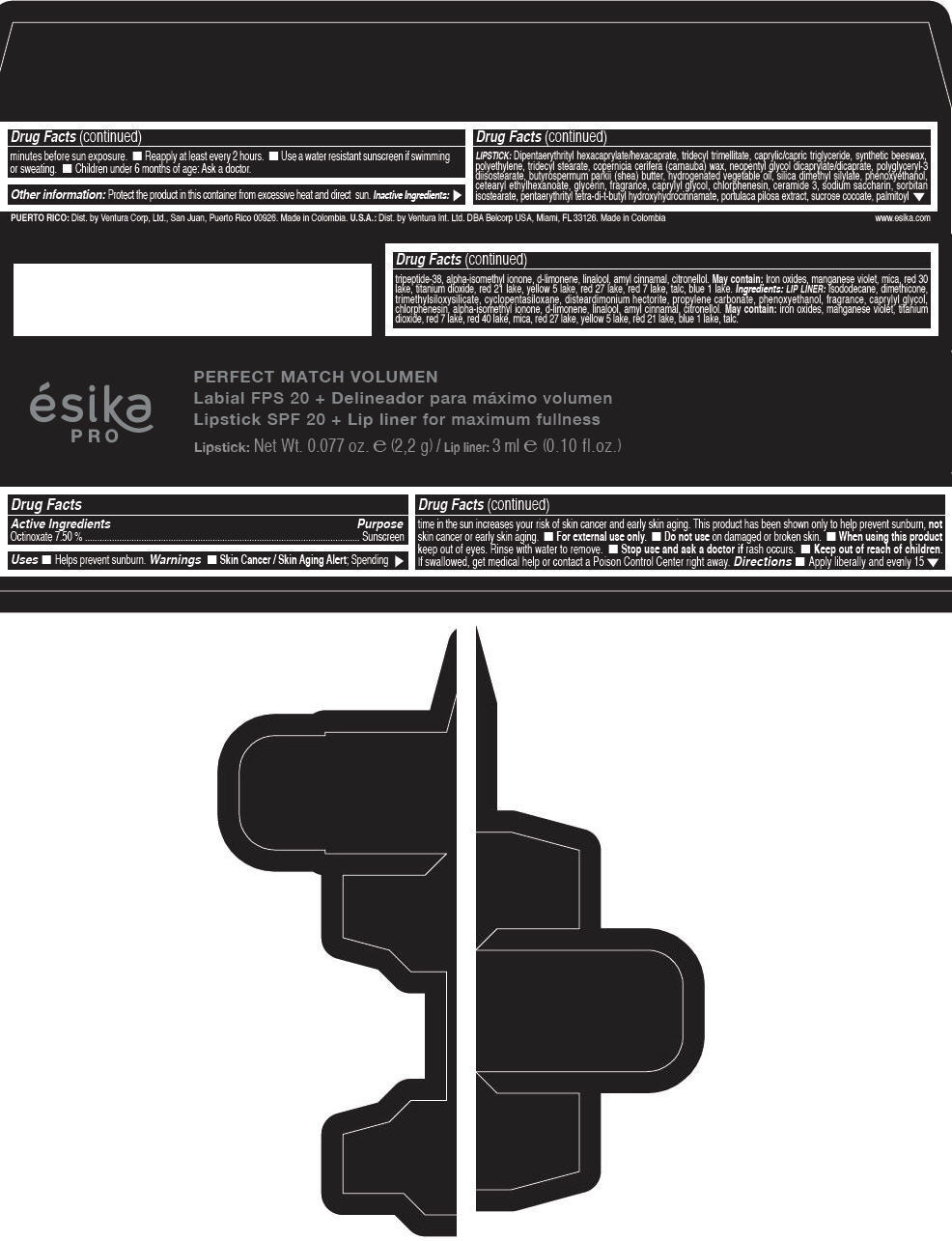
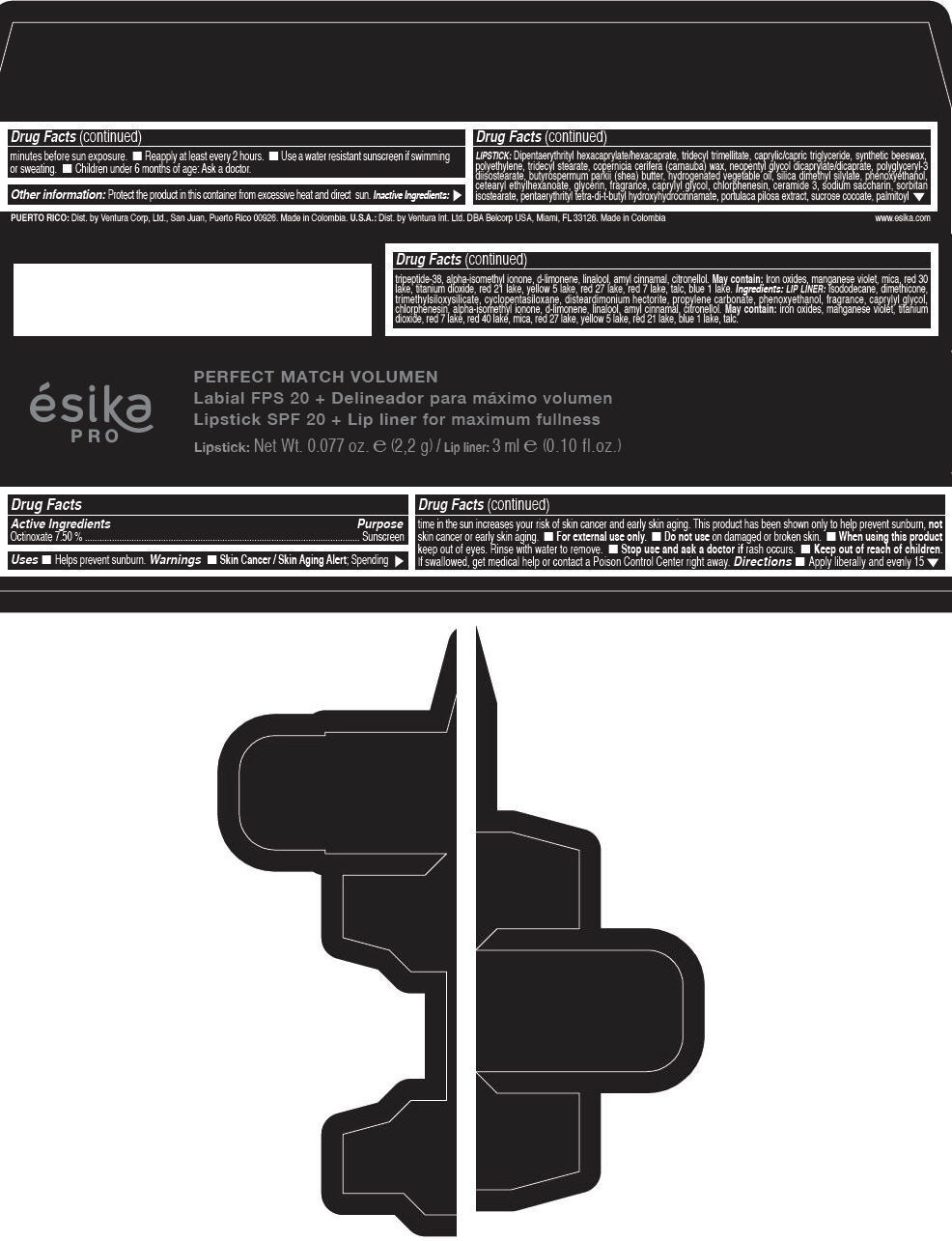
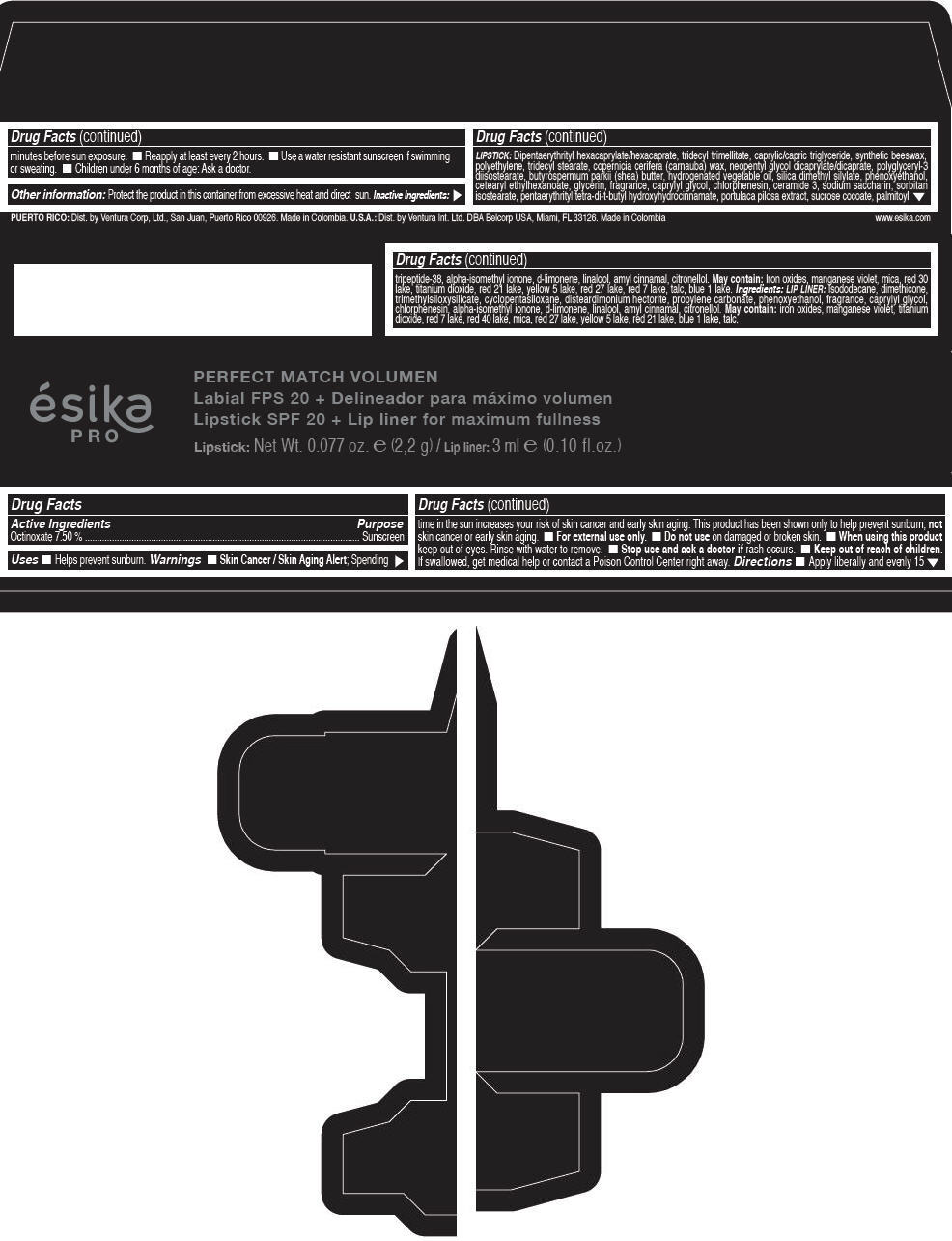
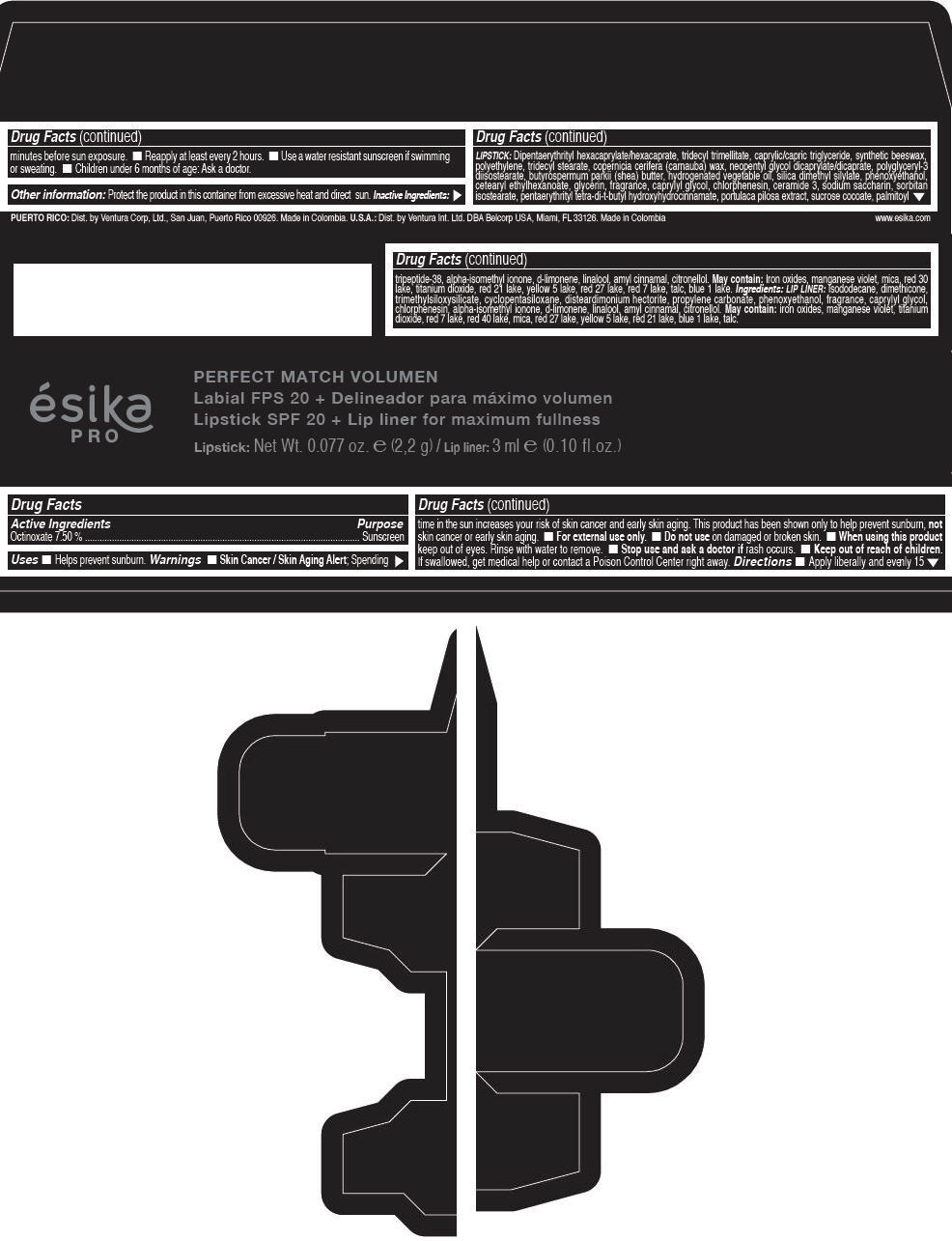
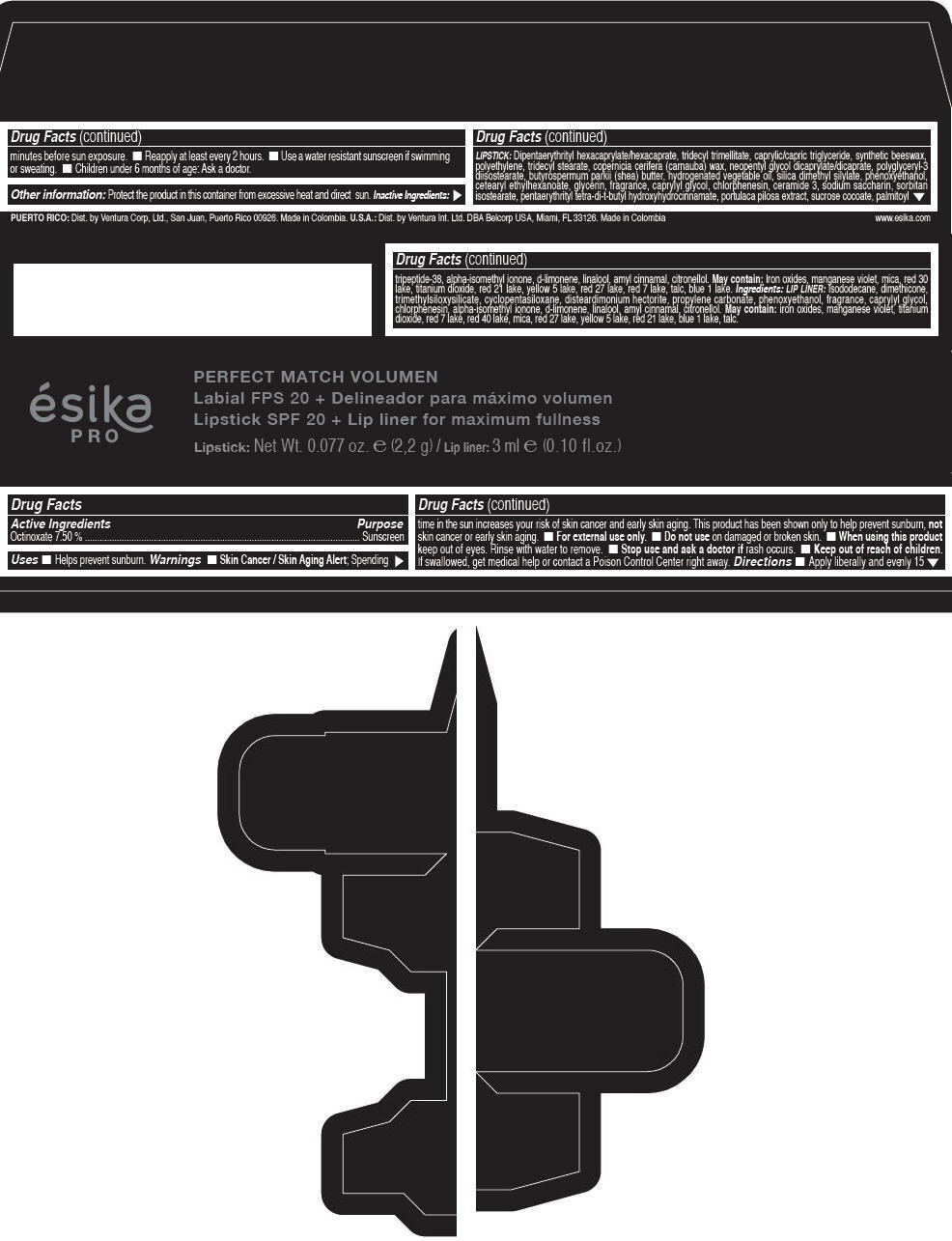
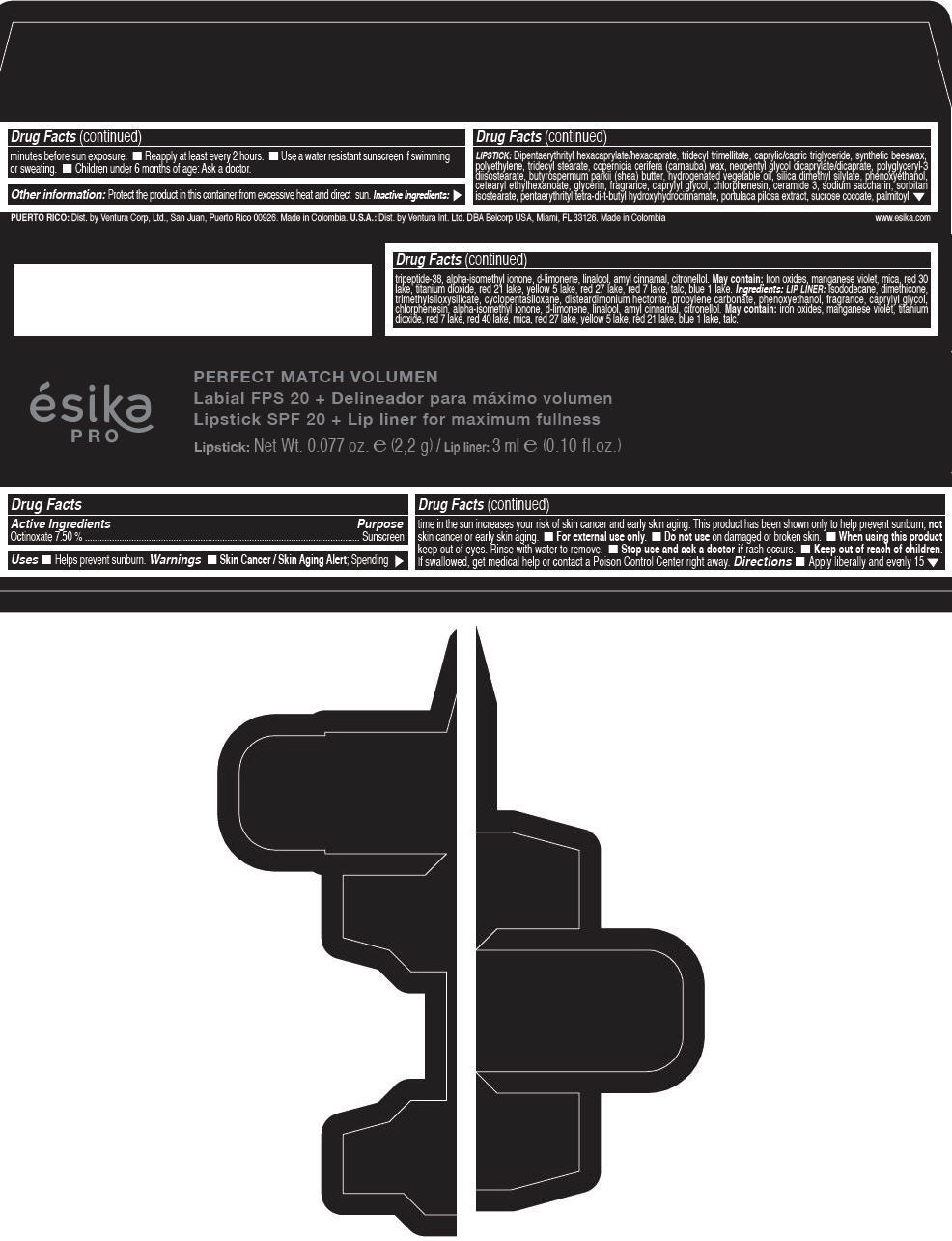
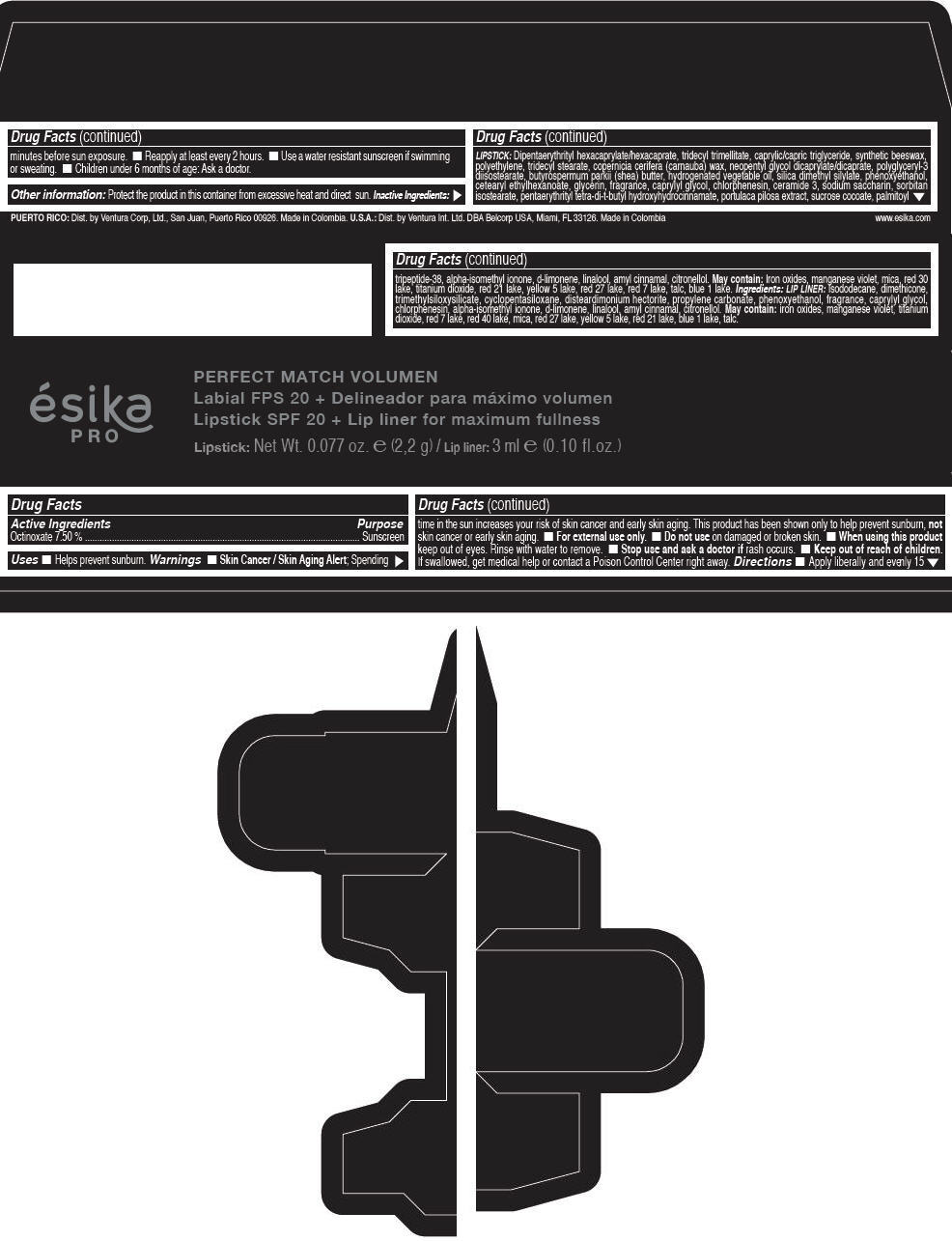
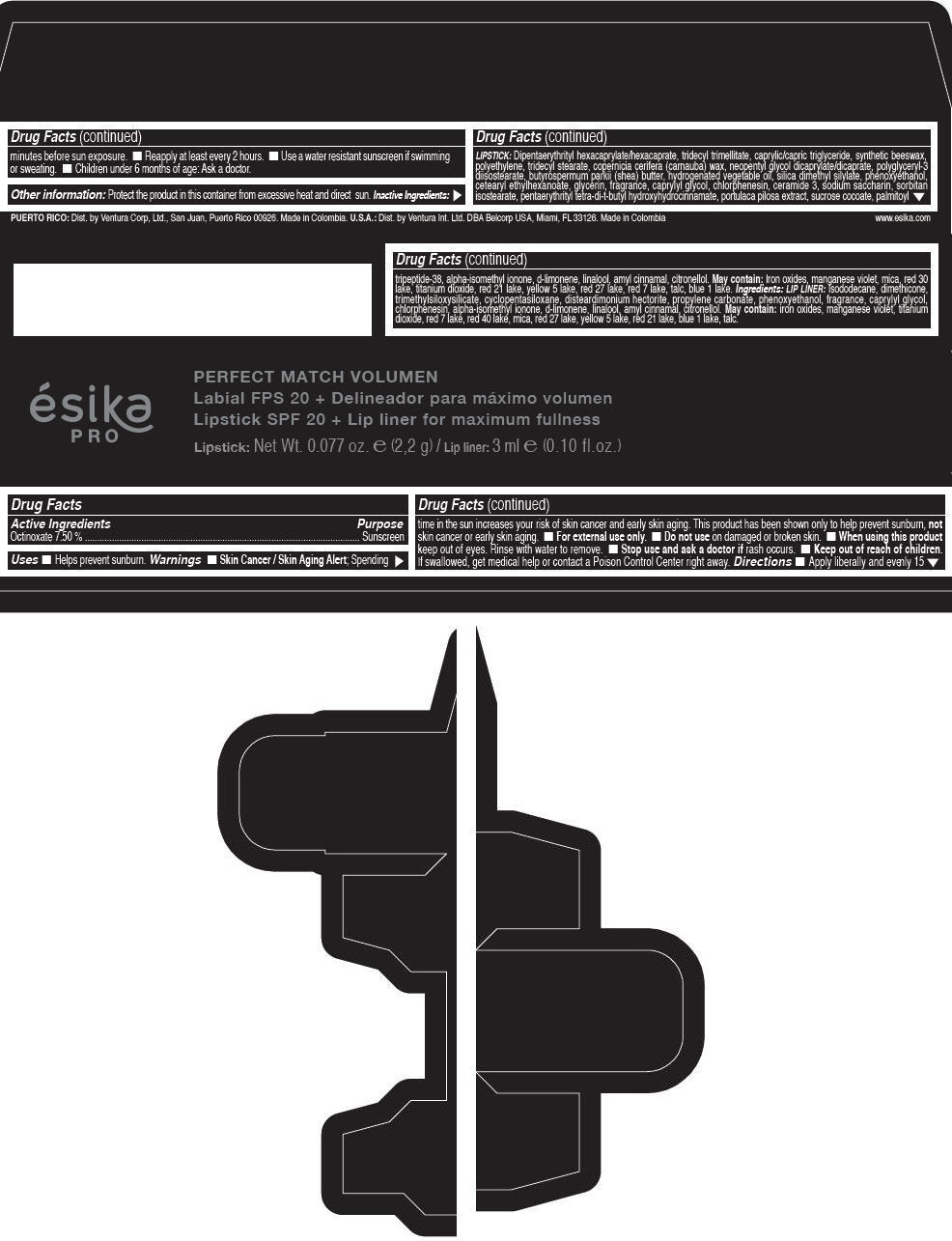
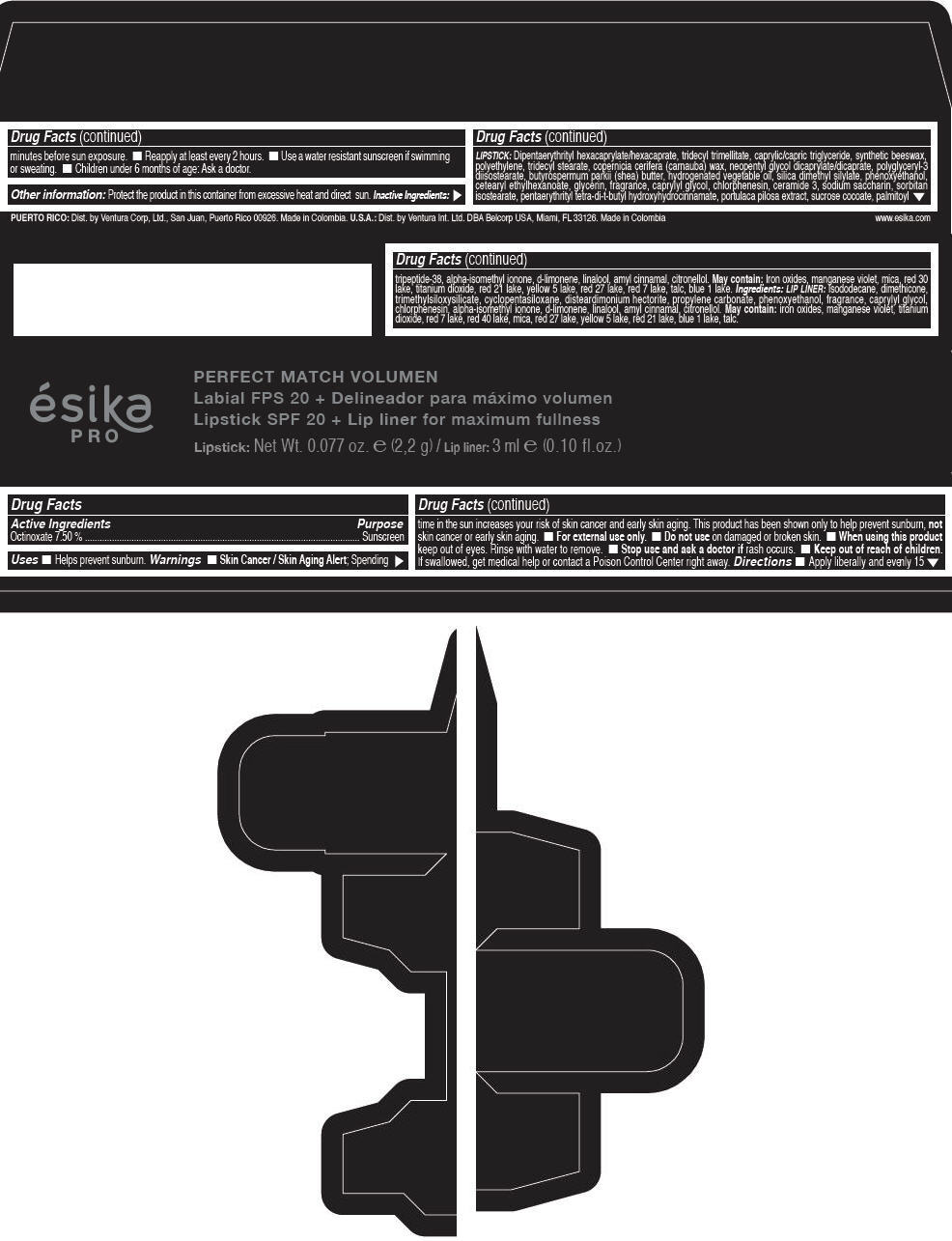
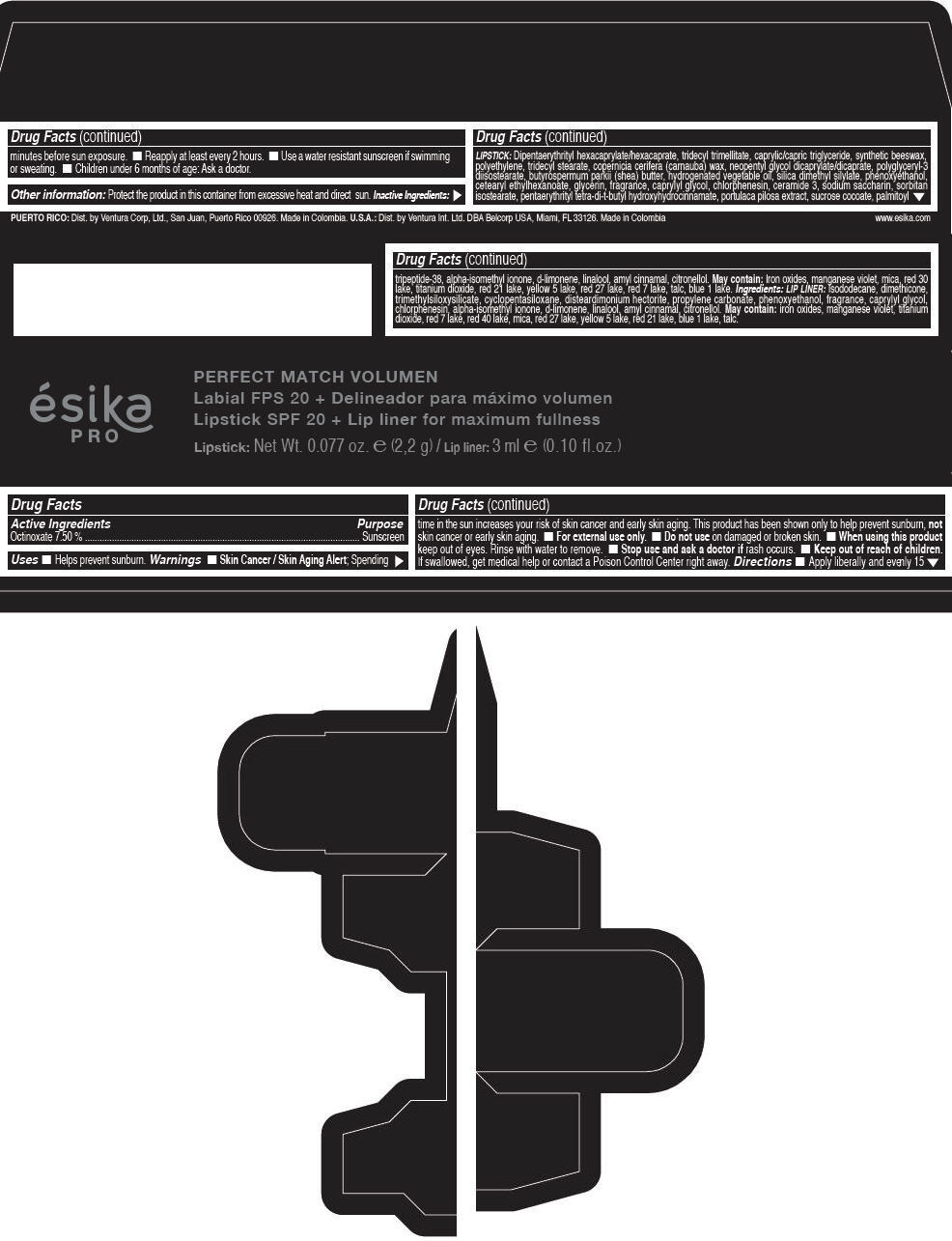
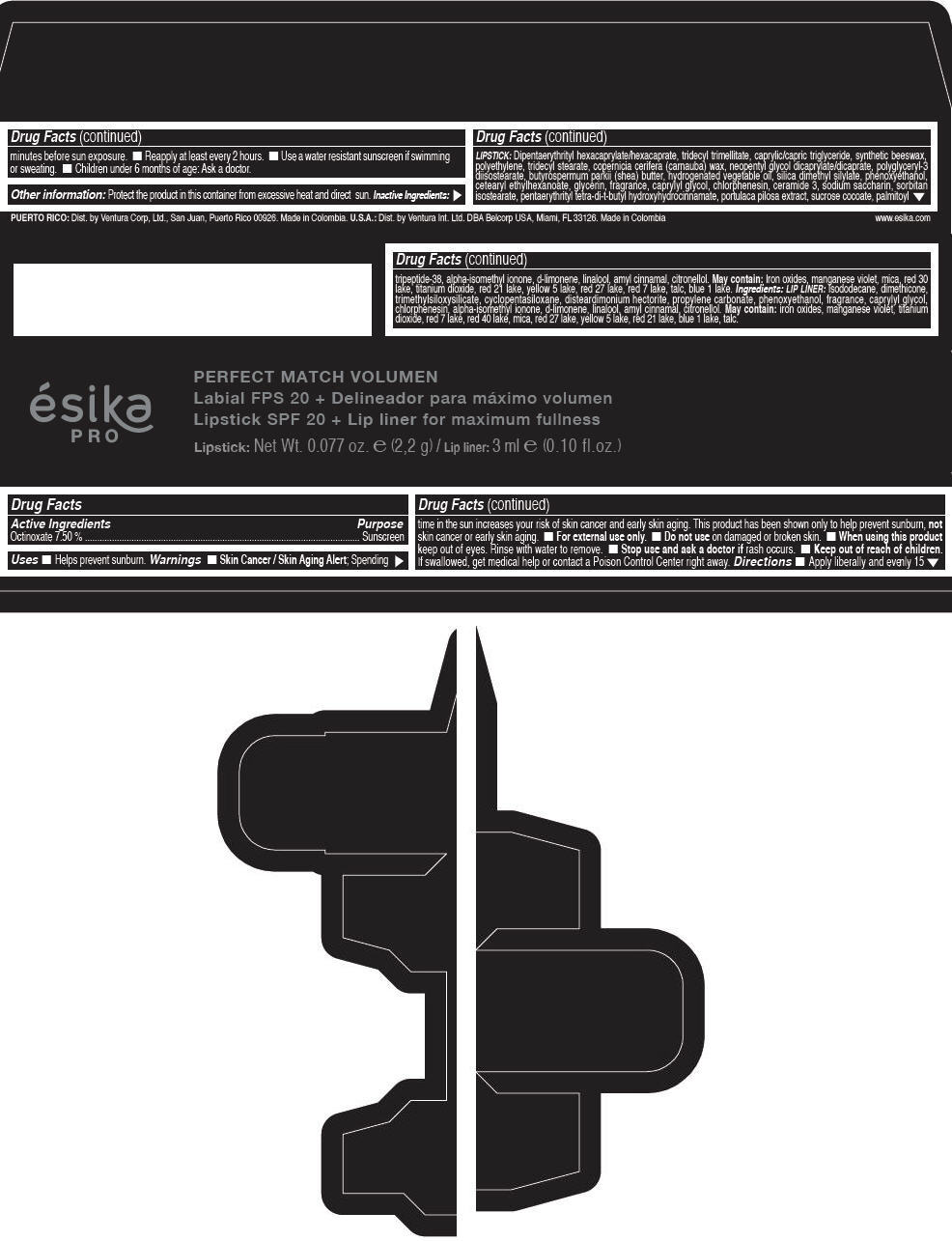
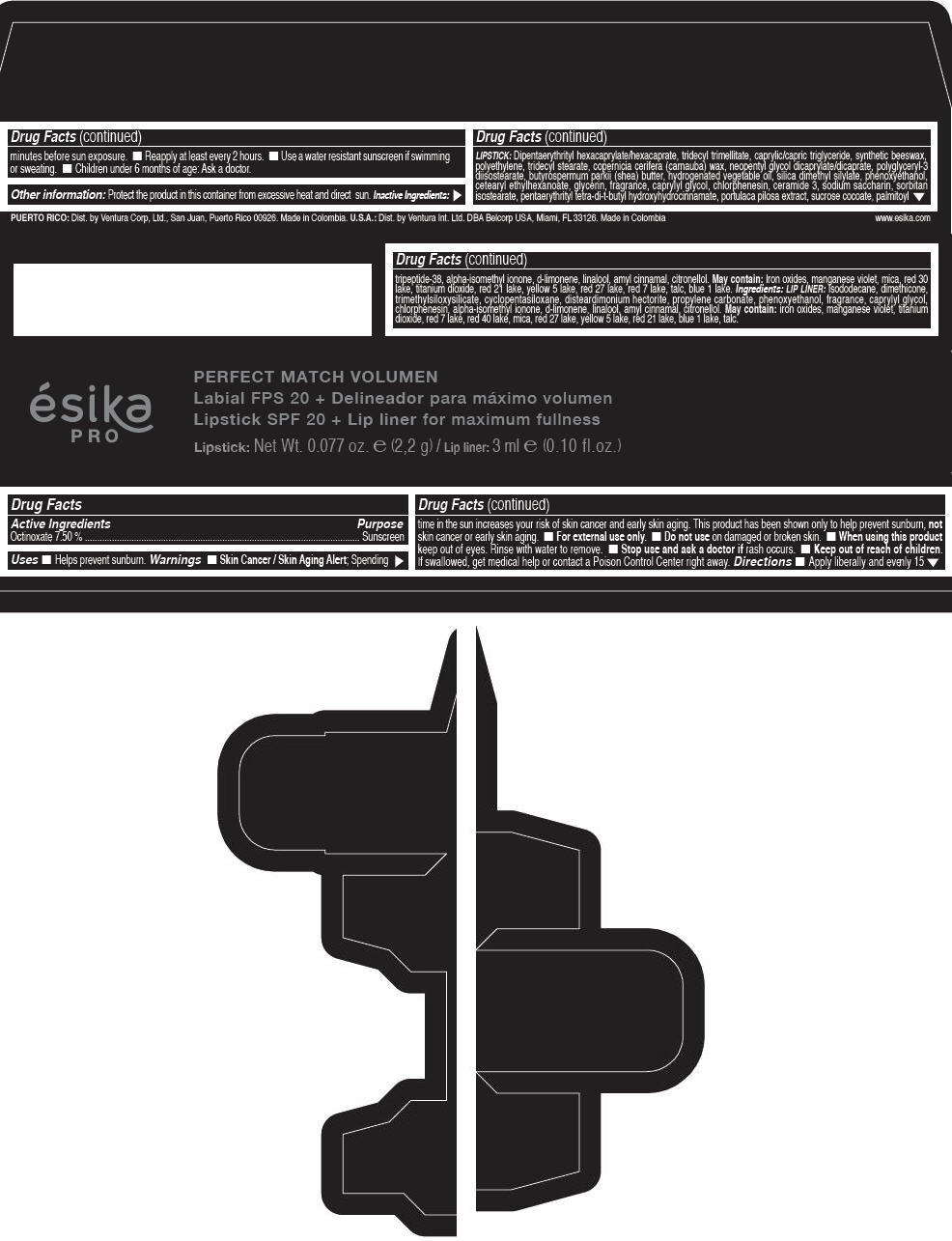
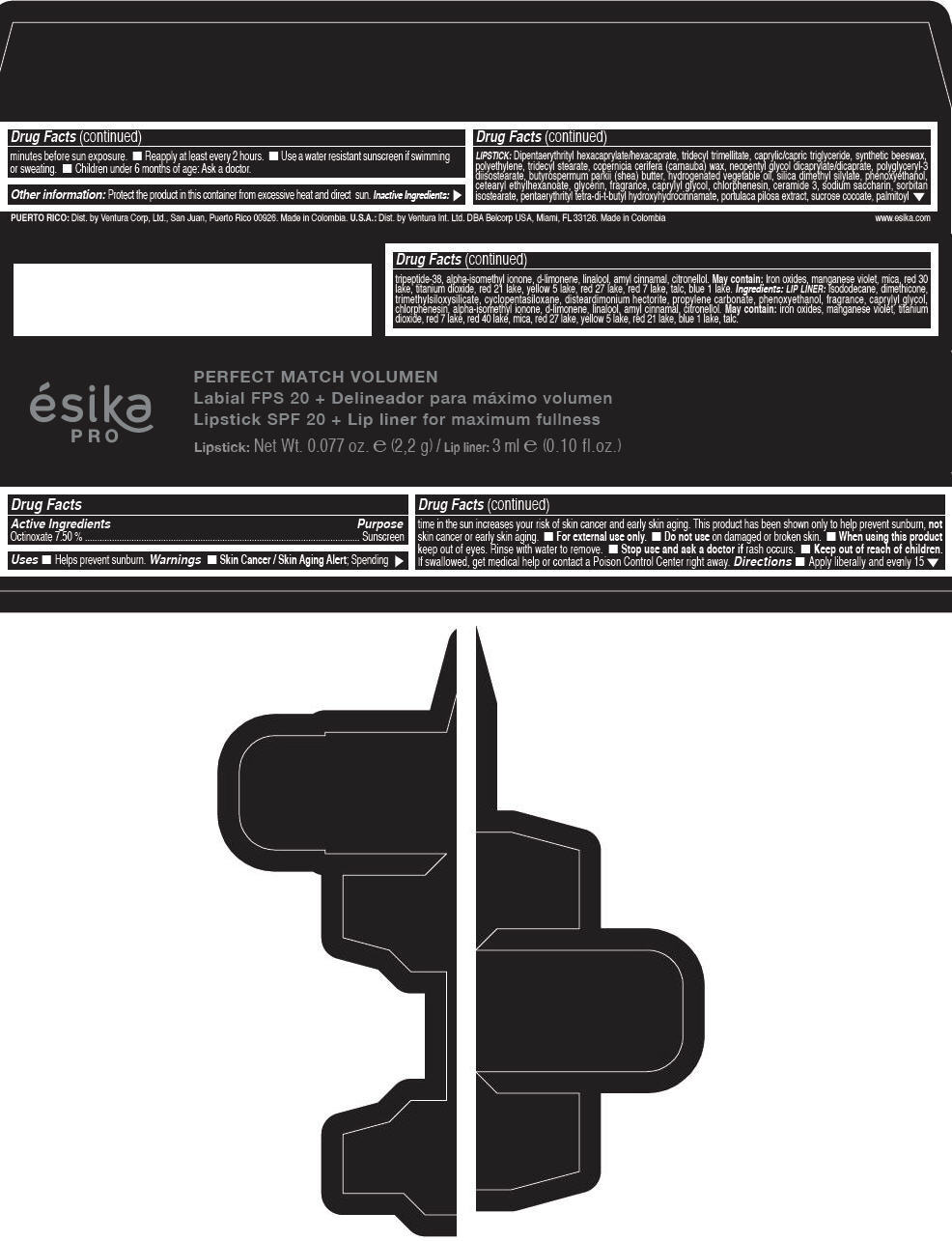
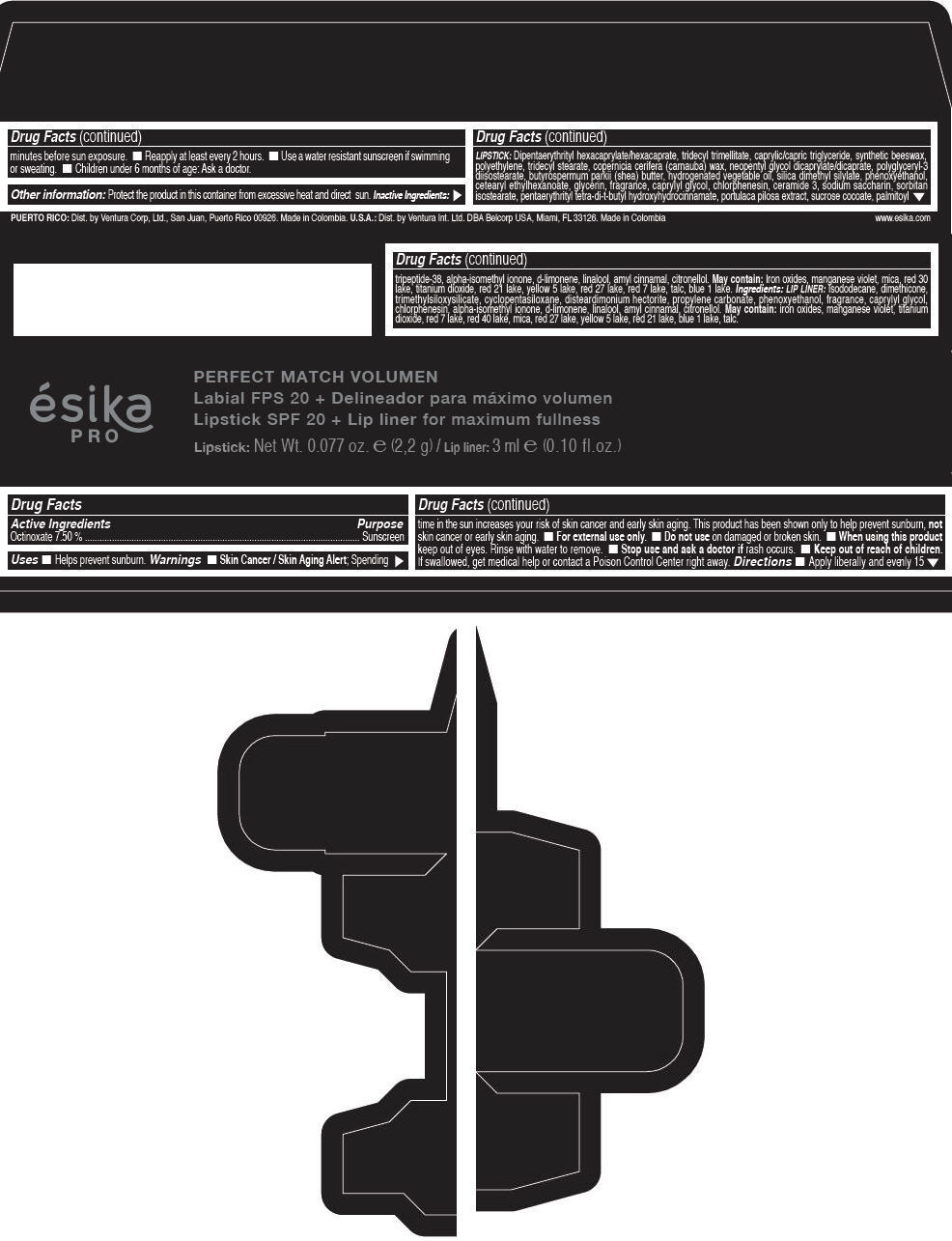
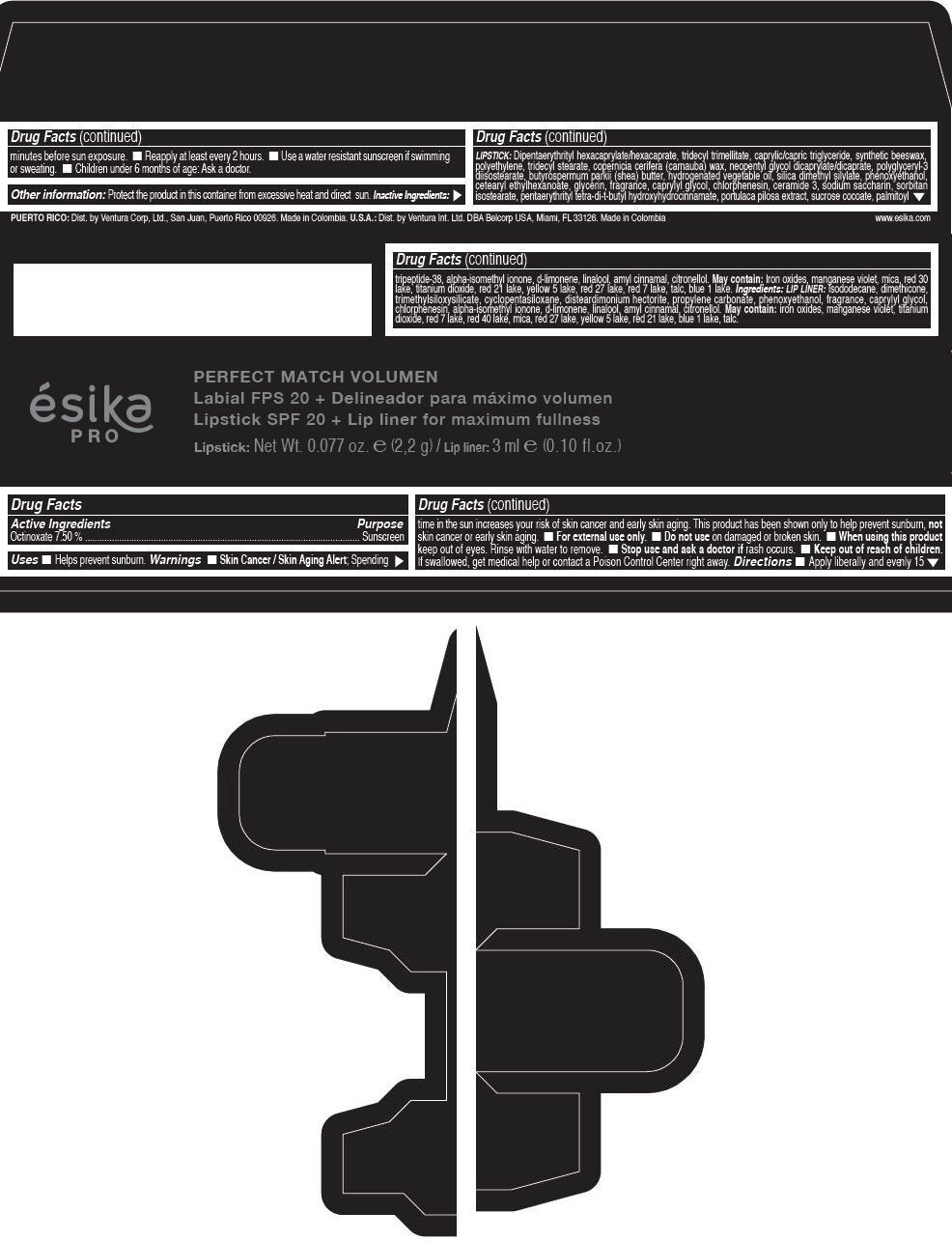
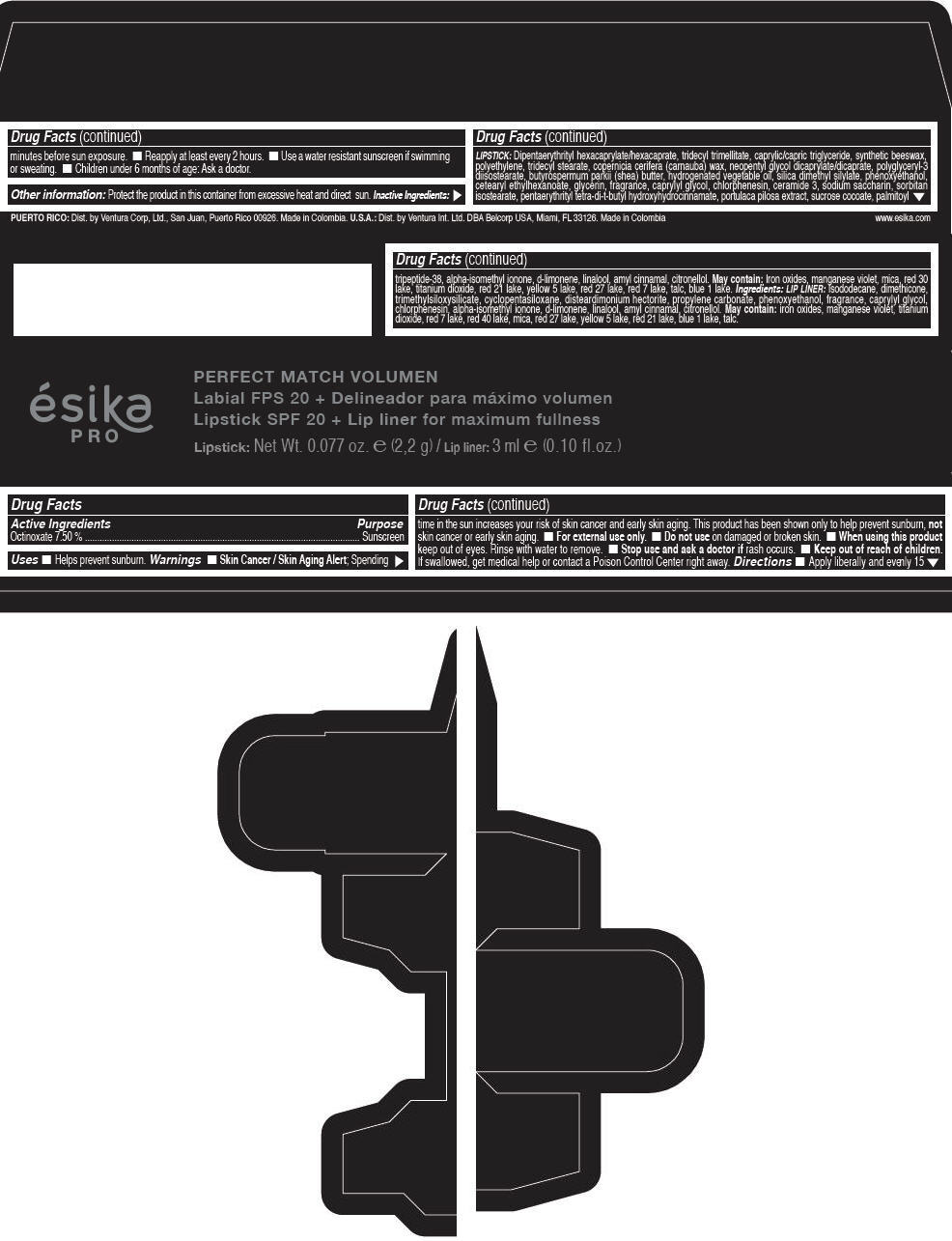
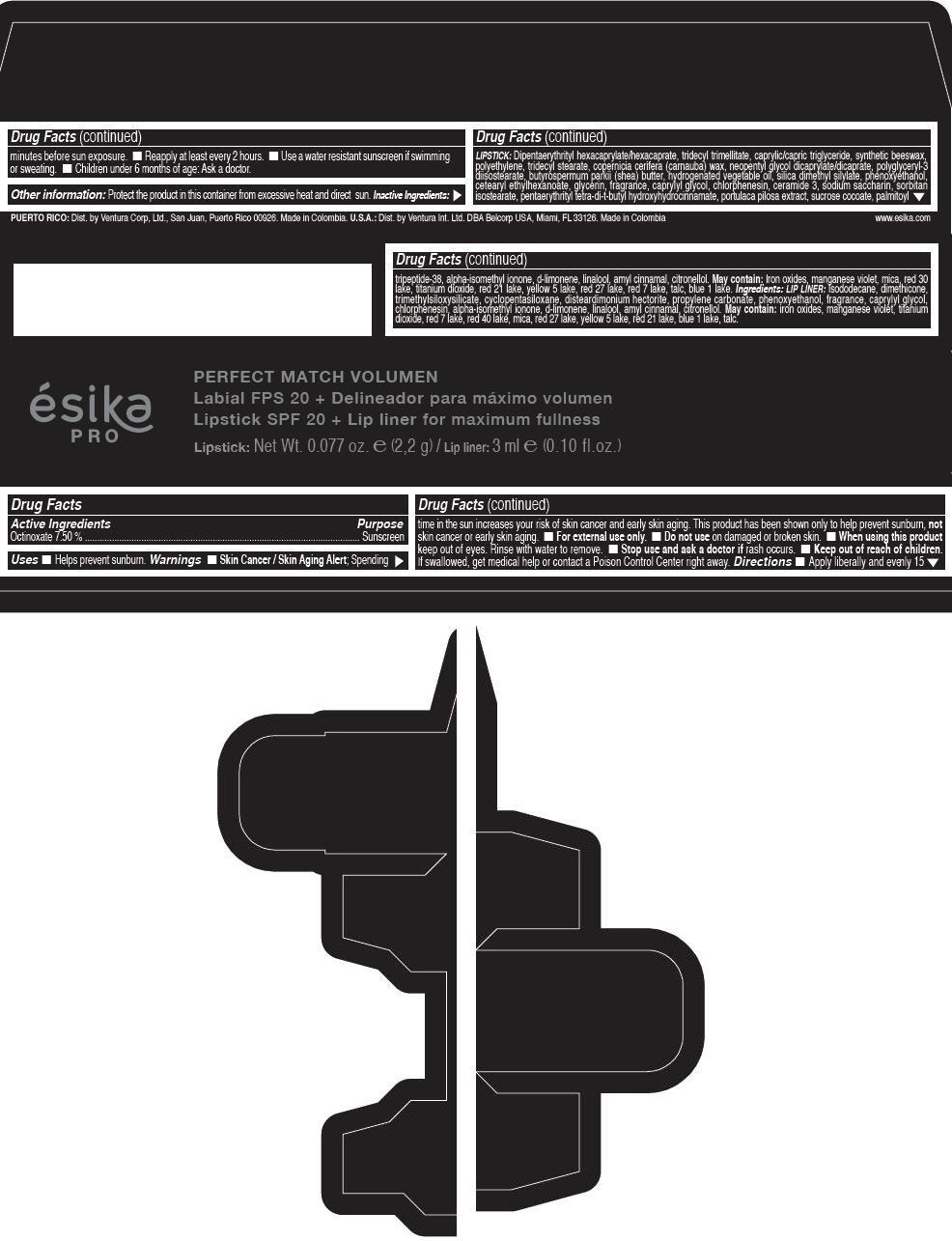
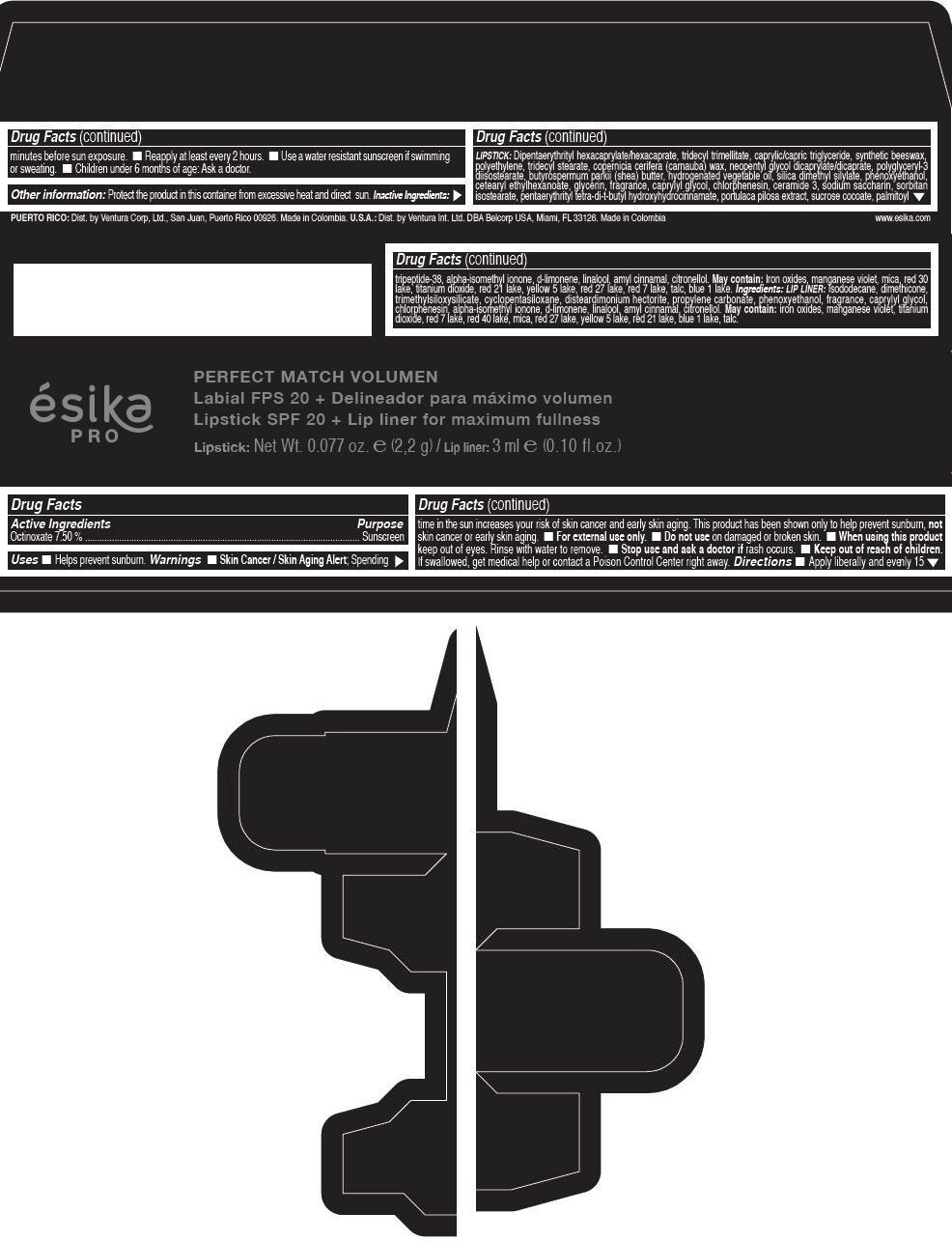
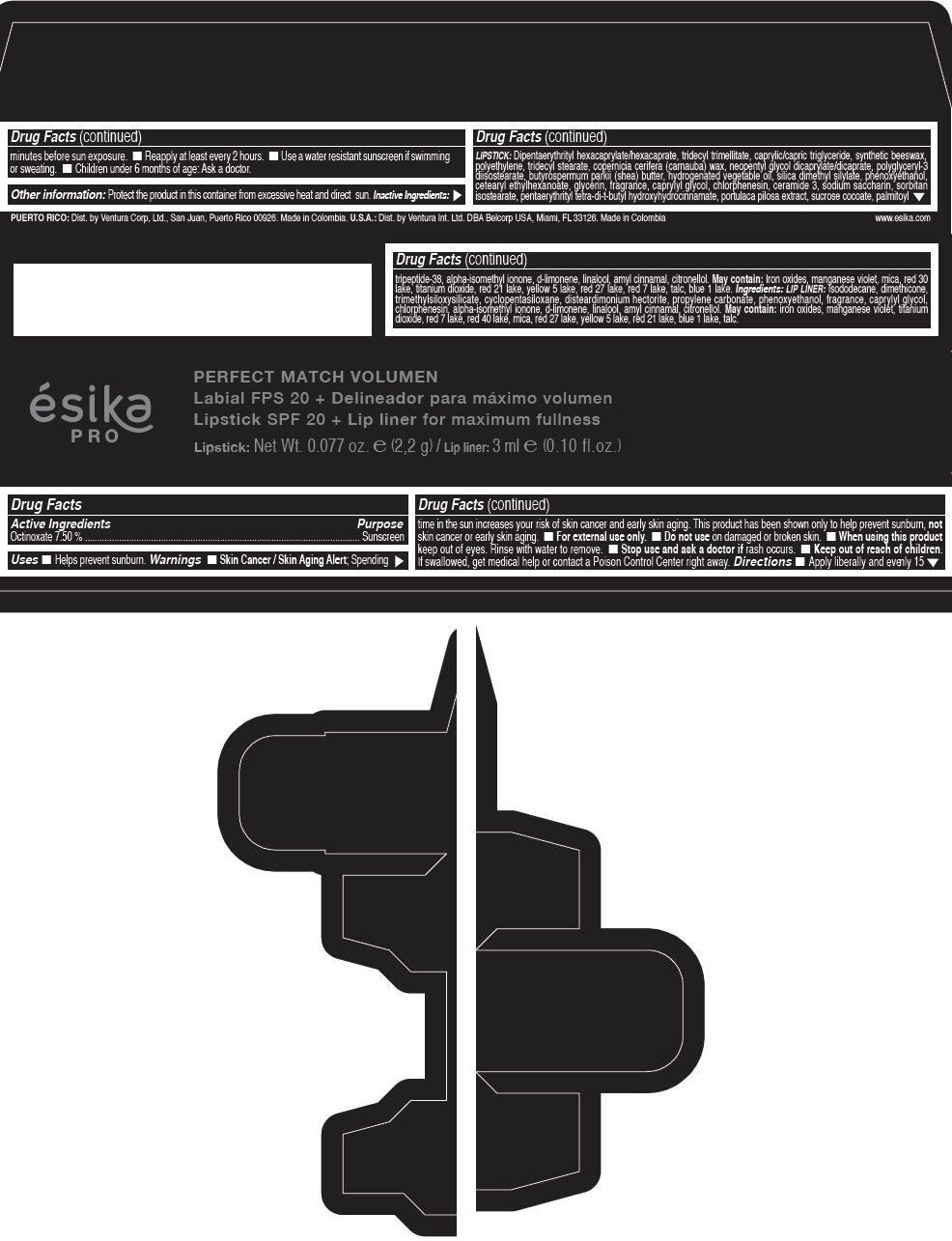
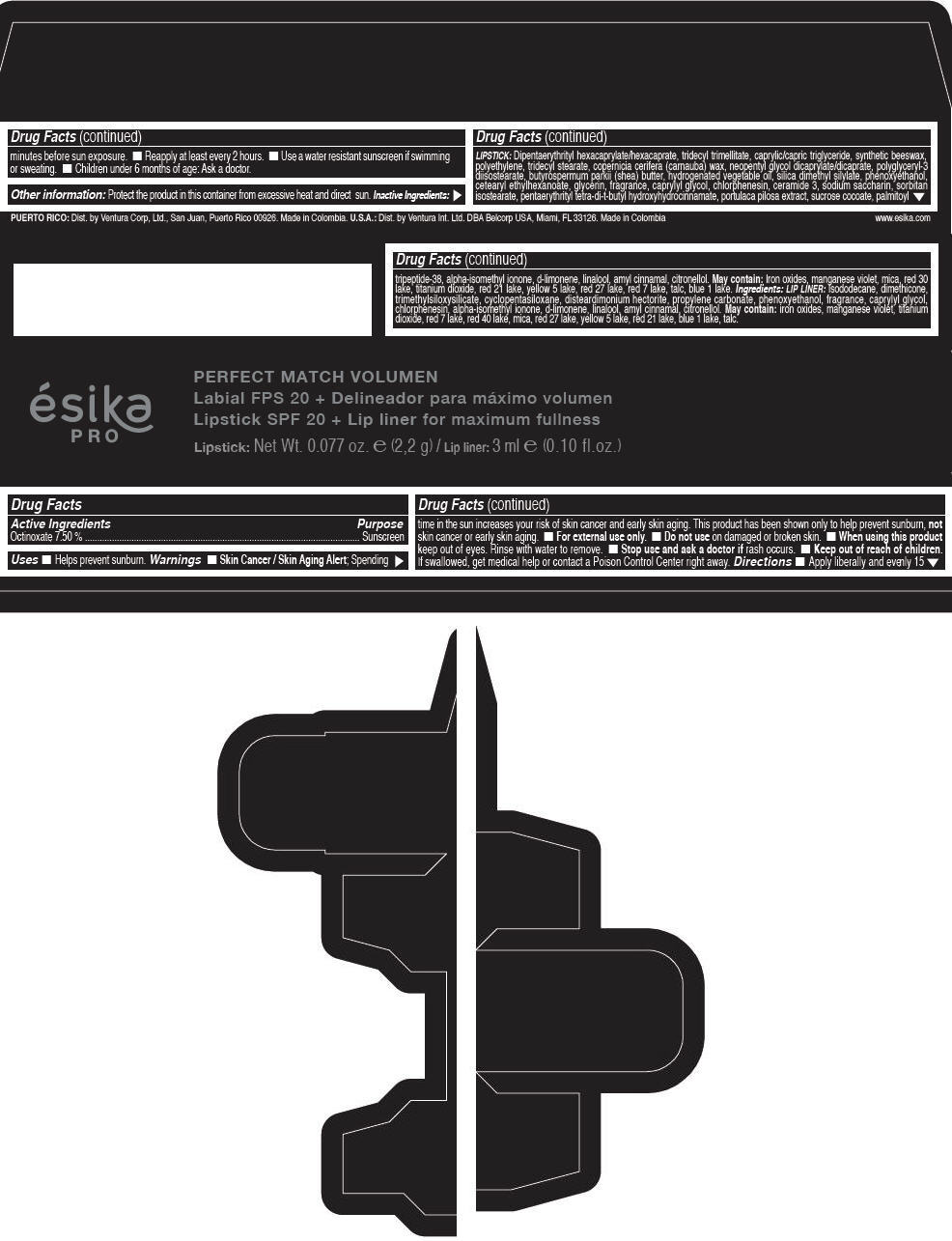
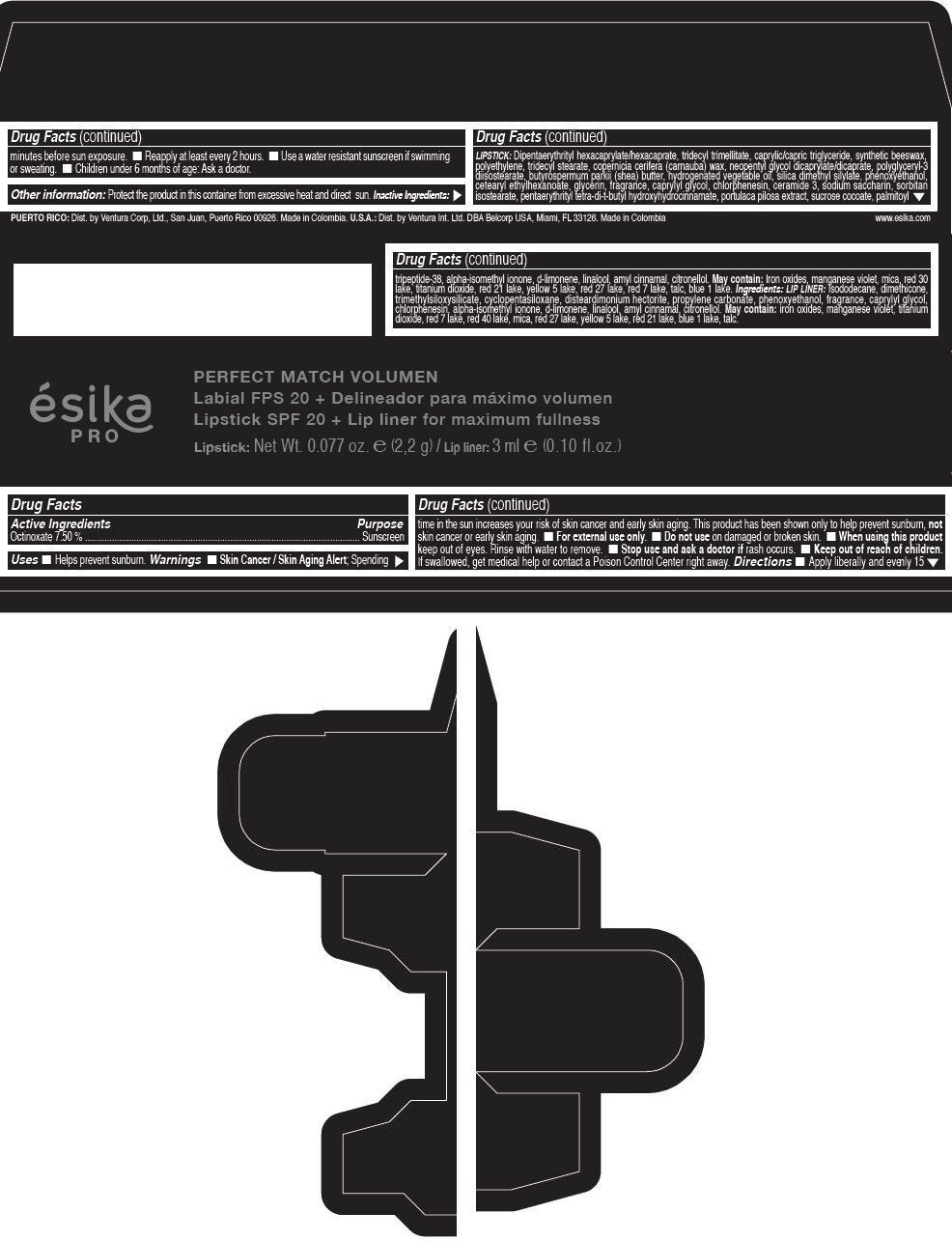
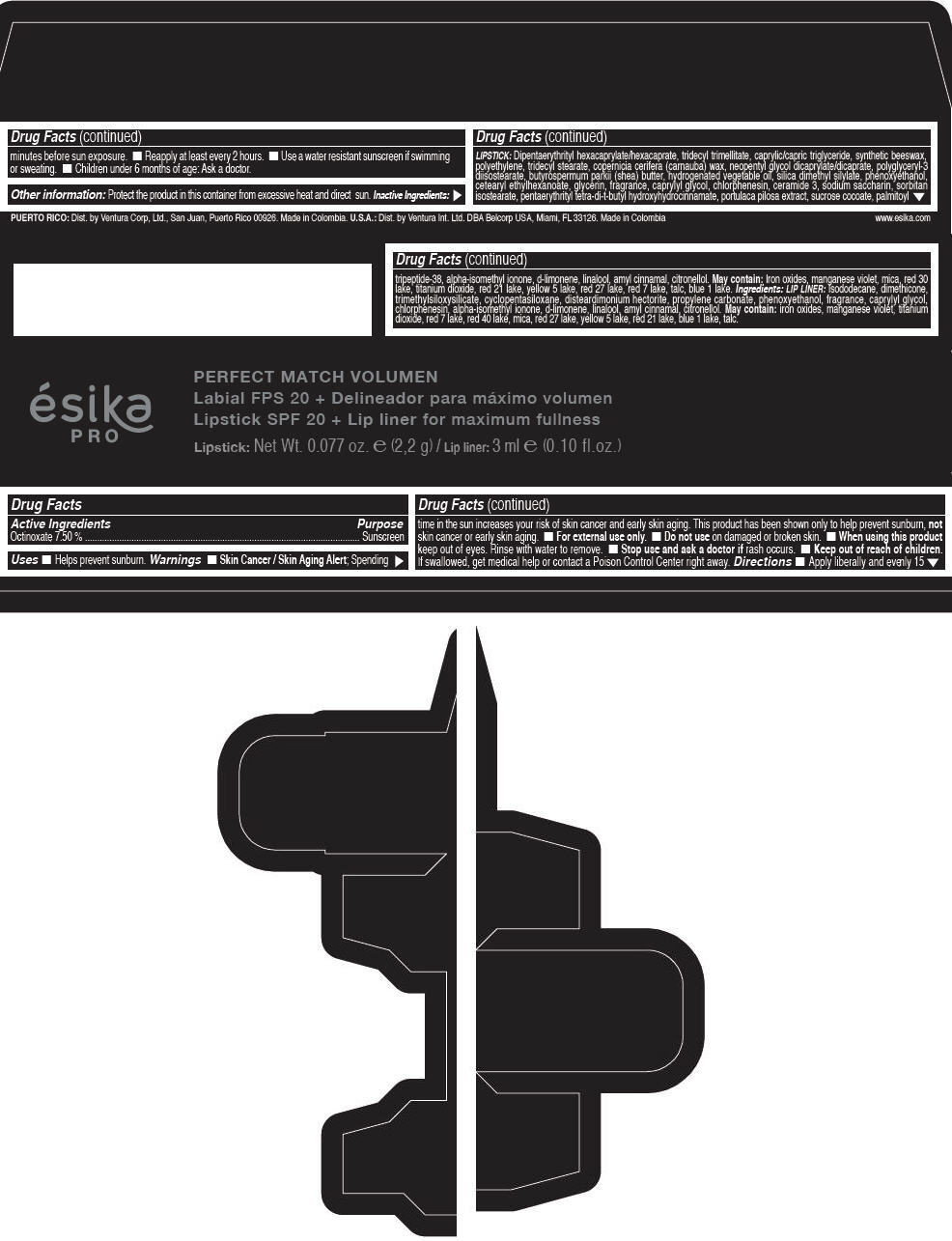
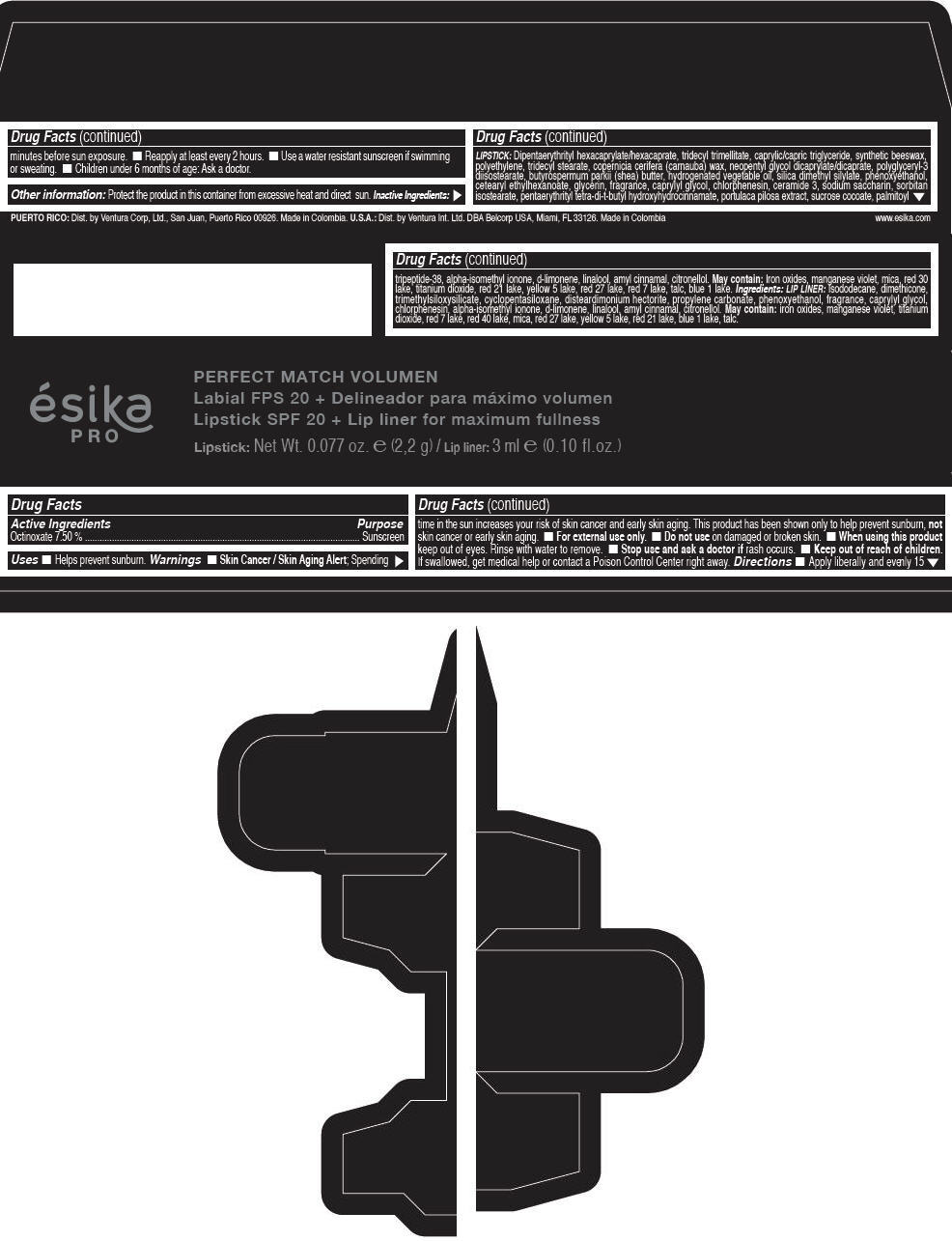
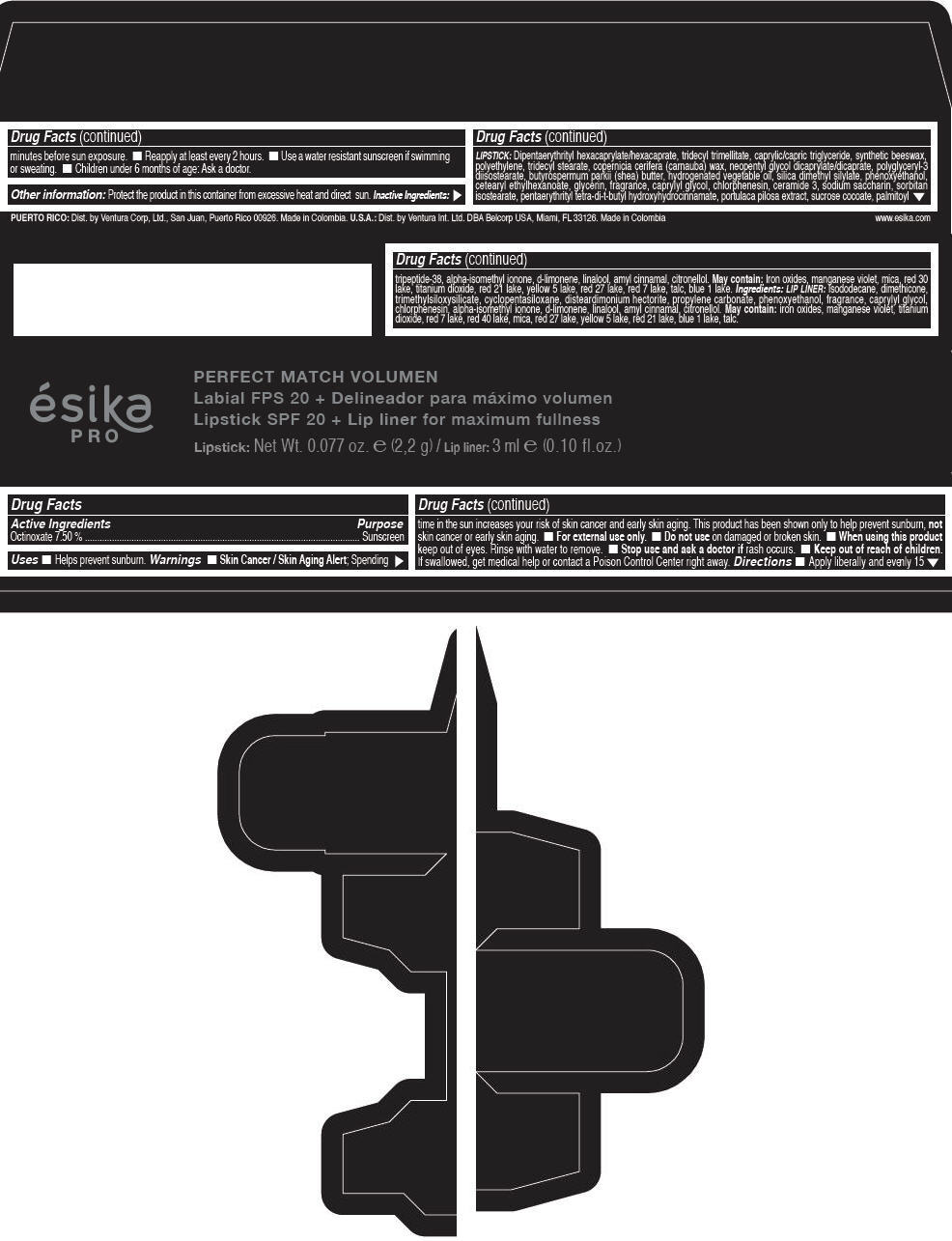
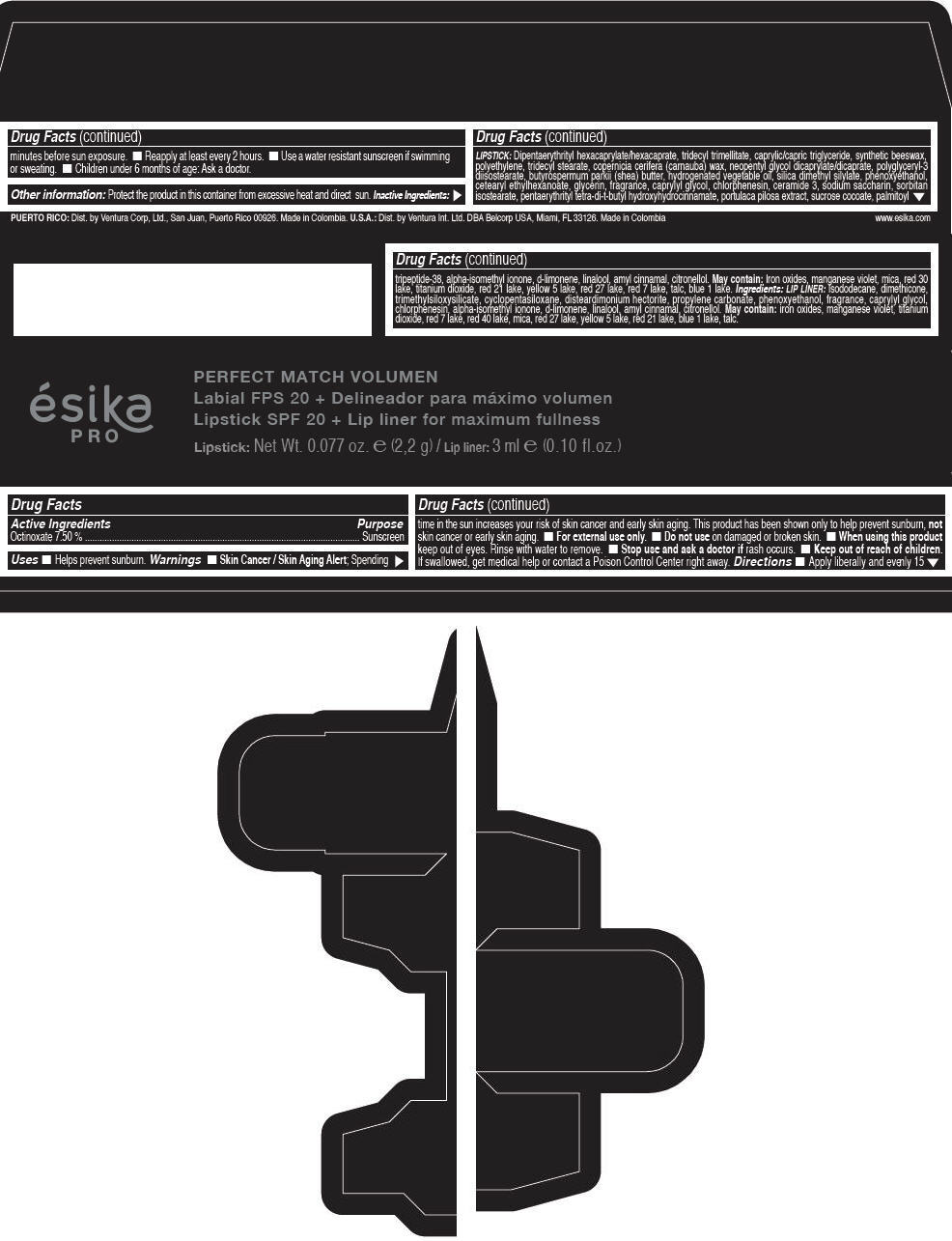
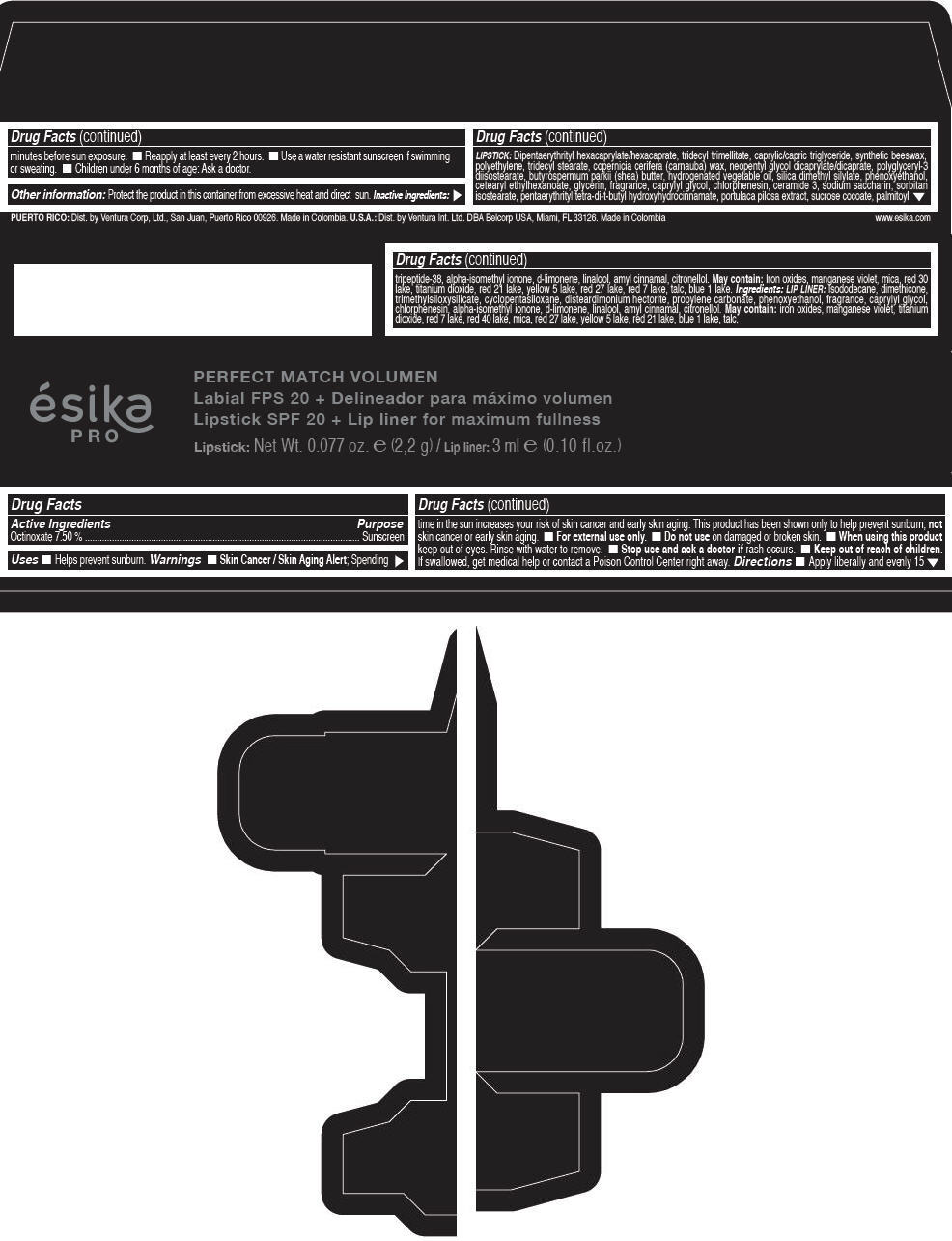
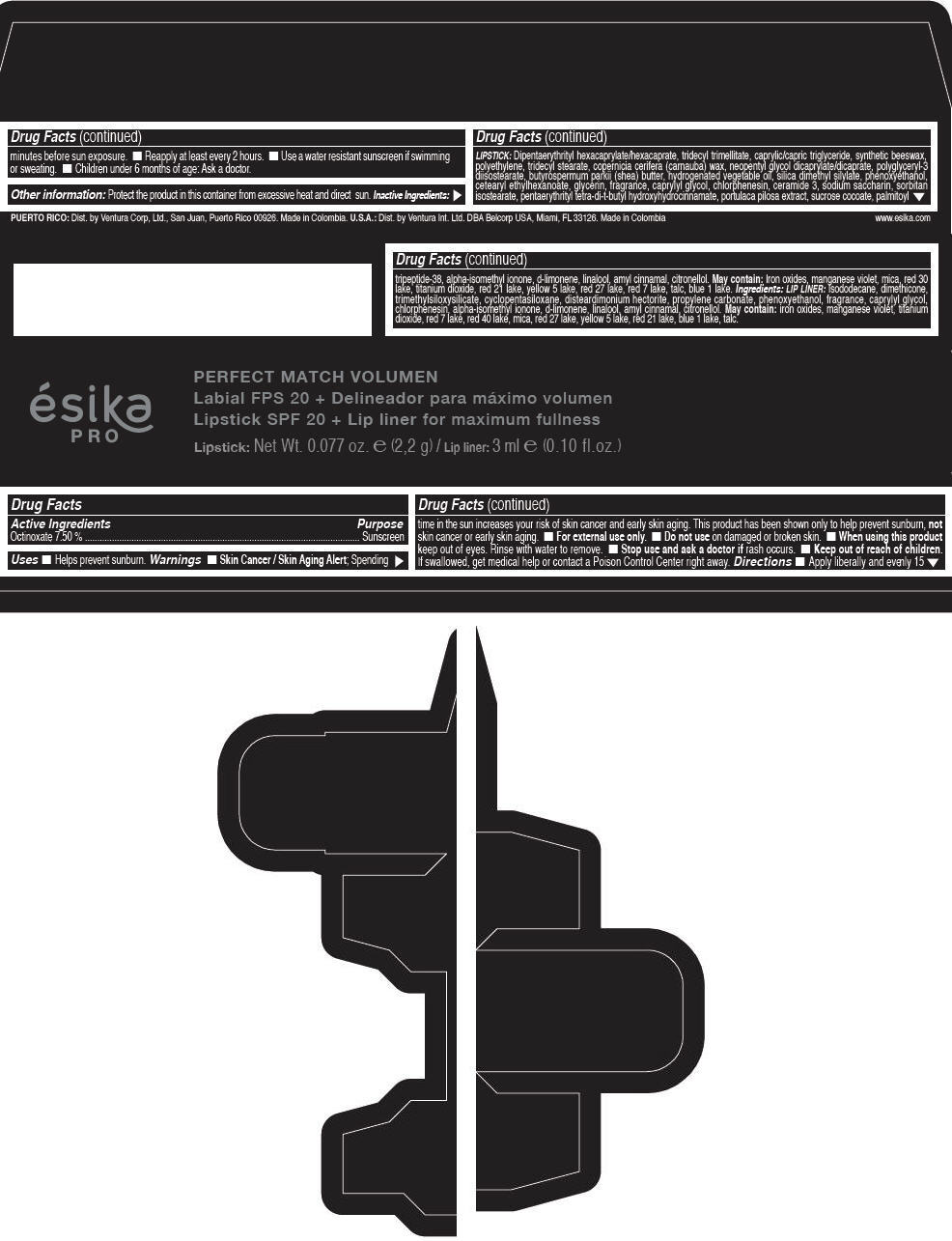
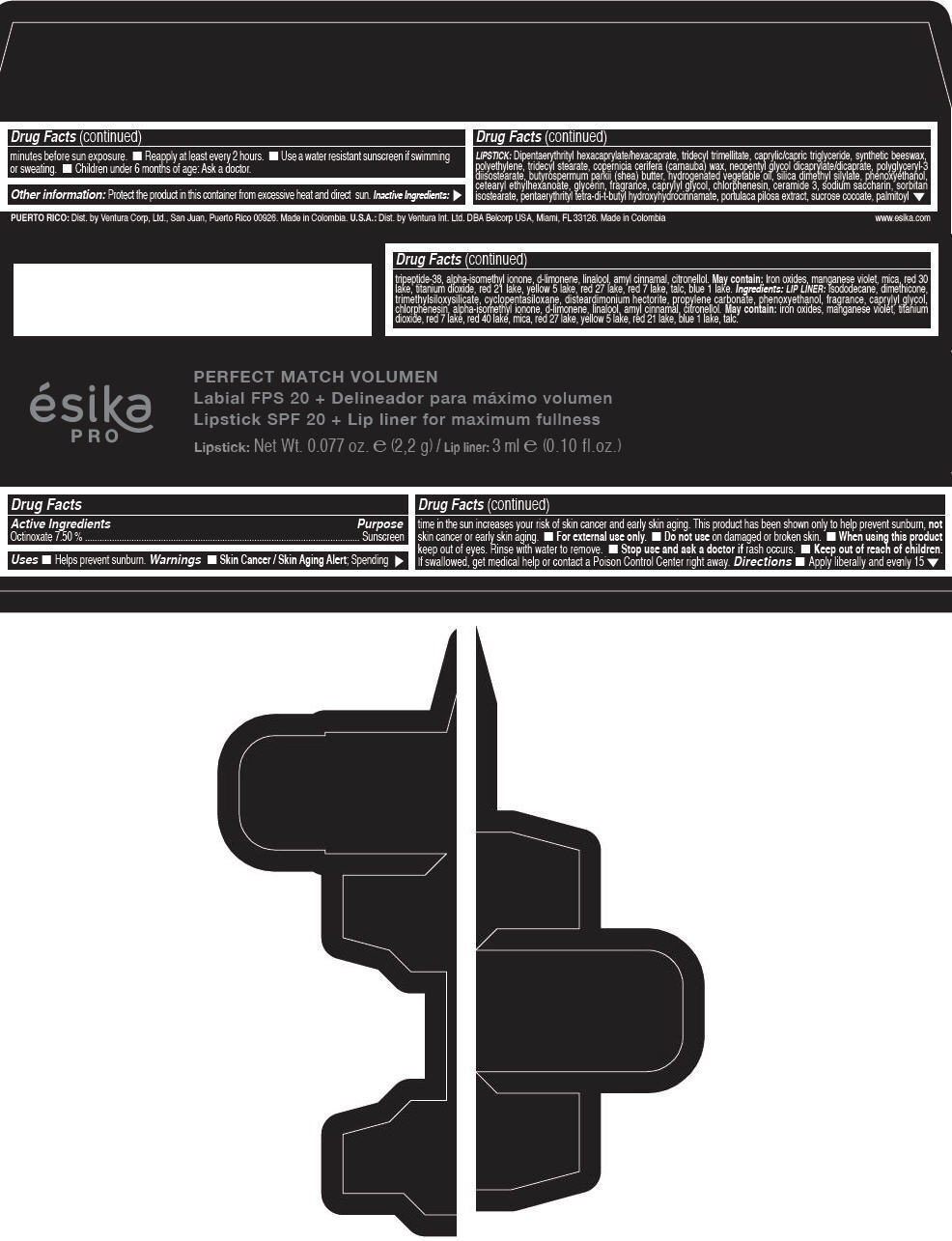
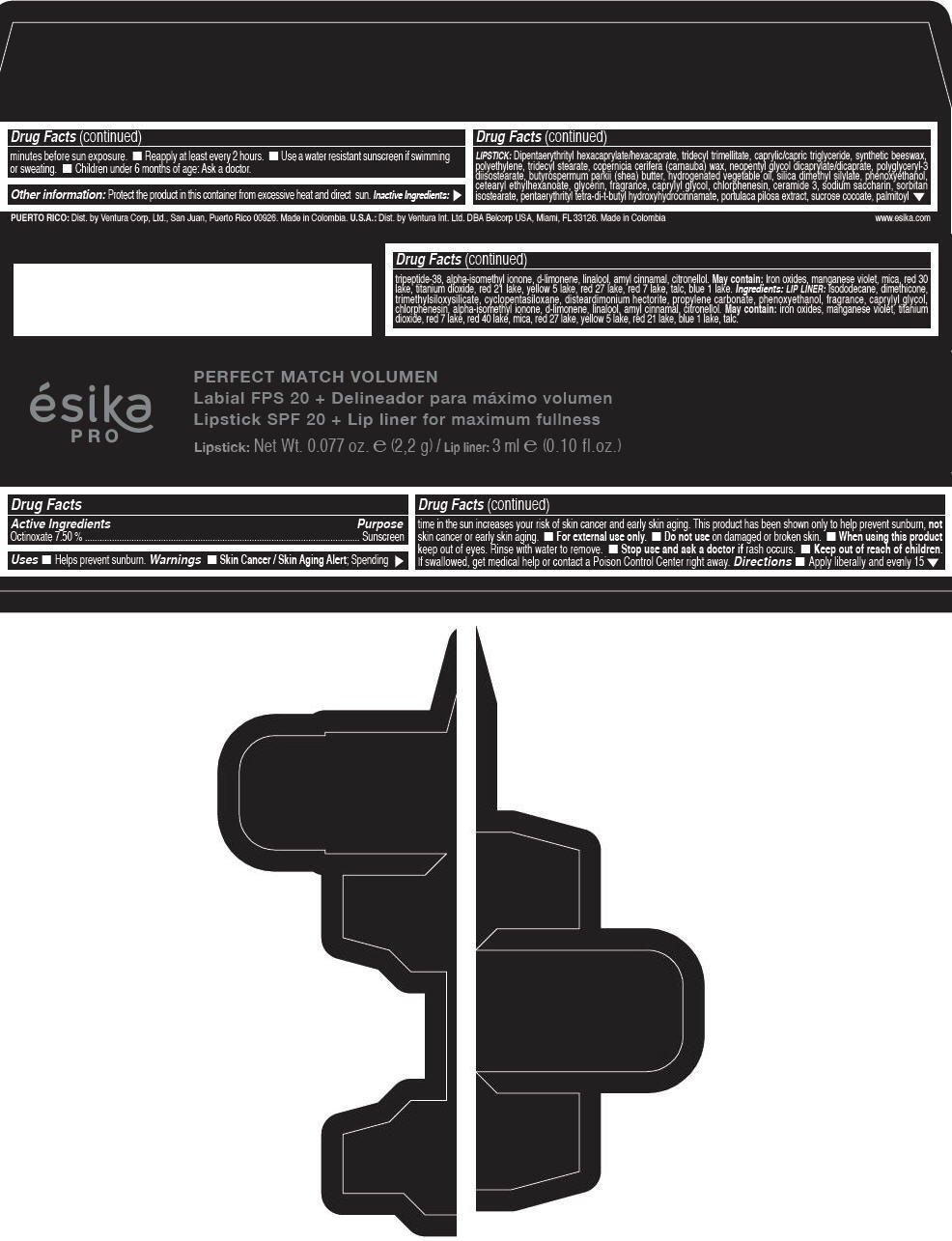
Label: ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (FRESA TENTACION) - RED- octinoxate lipstick
ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (CAFE MISTERIO) - BROWN- octinoxate lipstick
ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (ROSA DESEO) - BROWN- octinoxate lipstick
ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (BURGUNDY VITAL) - RED- octinoxate lipstick
ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (VINO SUBLIME) - RED- octinoxate lipstick
ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (ROJO RADIANT) - RED- octinoxate lipstick
-
Contains inactivated NDC Code(s)
NDC Code(s): 14783-113-01, 14783-113-02, 14783-114-01, 14783-114-02, view more14783-115-01, 14783-115-02, 14783-116-01, 14783-116-02, 14783-117-01, 14783-117-02, 14783-118-01, 14783-118-02, 14783-822-01, 14783-822-02, 14783-823-01, 14783-823-02, 14783-824-01, 14783-824-02, 14783-825-01, 14783-825-02, 14783-826-01, 14783-826-02, 14783-827-01, 14783-827-02, 14783-828-01, 14783-828-02, 14783-829-01, 14783-829-02, 14783-830-01, 14783-830-02, 14783-831-01, 14783-831-02, 14783-832-01, 14783-832-02 - Packager: Ventura International LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 6, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
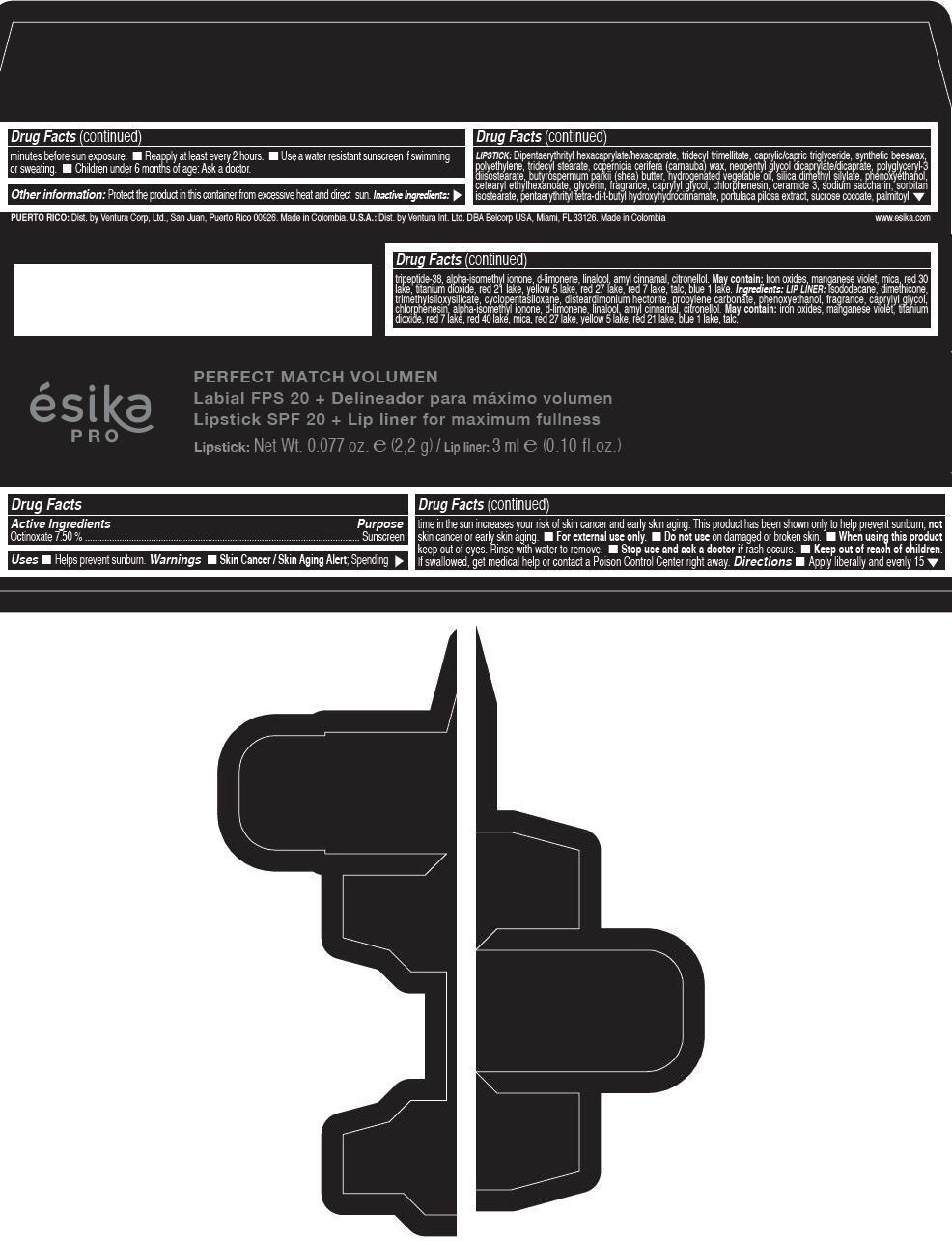
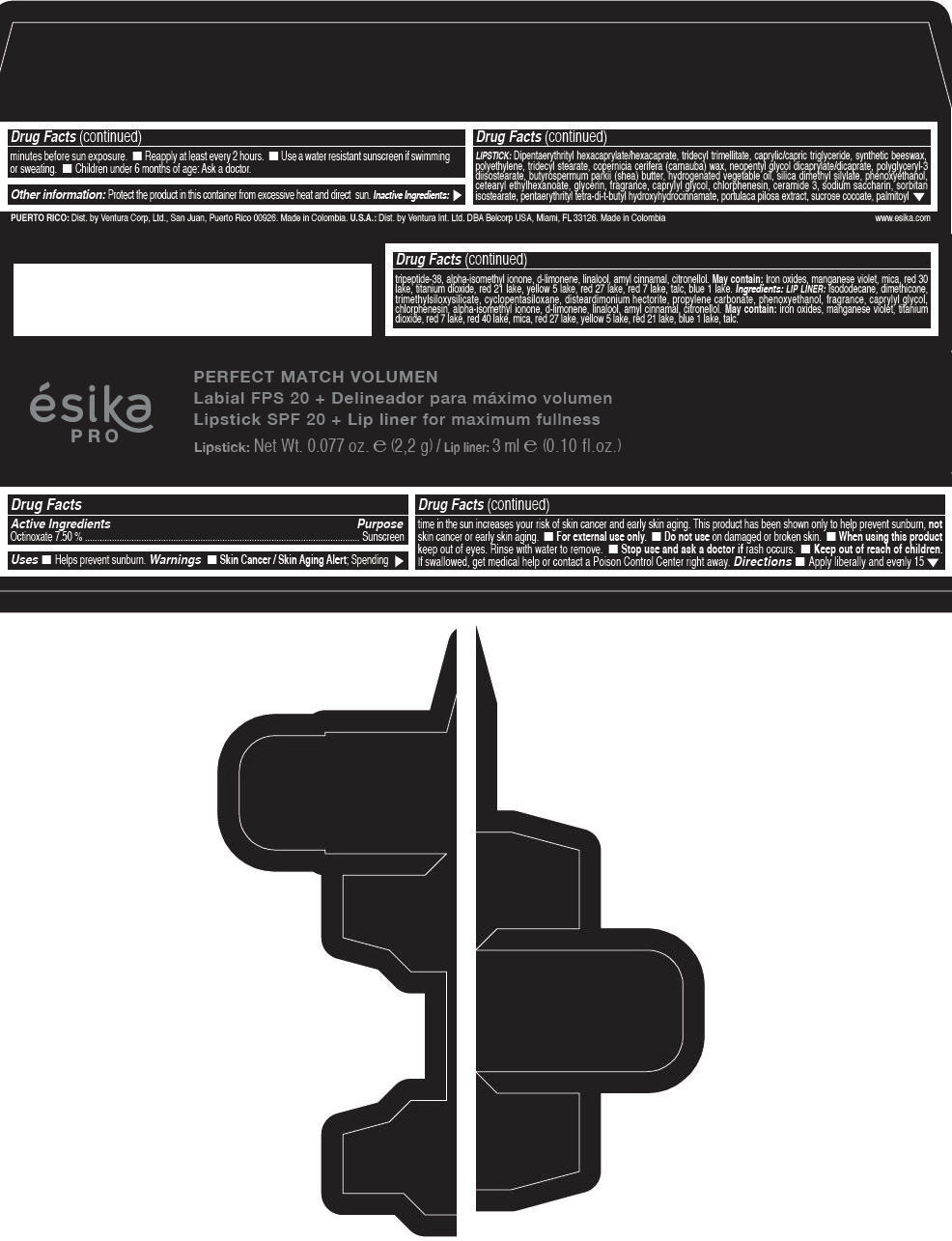
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
LIPSTICK
DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE, TRIDECYL TRIMELLITATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, SYNTHETIC BEESWAX, POLYETHYLENE, TRIDECYL STEARATE, COPERNICIA CERIFERA (CARNAUBA) WAX, NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE, POLYGLYCERYL-3 DIISOSTEARATE, BUTYROSPERMUM PARKII (SHEA) BUTTER, HYDROGENATED VEGETABLE OIL, SILICA DIMETHYL SILYLATE, PHENOXYETHANOL, CETEARYL ETHYLHEXANOATE, GLYCERIN, FRAGRANCE, CAPRYLYL GLYCOL, CHLORPHENESIN, CERAMIDE 3, SODIUM SACCHARIN, SORBITAN ISOSTEARATE, PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE, PORTULACA PILOSA EXTRACT, SUCROSE COCOATE, PALMITOYL TRIPEPTIDE-38, ALPHA-ISOMETHYL IONONE, d-LIMONENE, LINALOOL, AMYL CINNAMAL, CITRONELLOL.
MAY CONTAIN :
IRON OXIDES, MANGANESE VIOLET, MICA, RED 30 LAKE, TITANIUM DIOXIDE, RED 21 LAKE, YELLOW 5 LAKE, RED 27 LAKE, RED 7 LAKE, TALC, BLUE 1 LAKE.
-
INGREDIENTS
LIP LINER
ISODODECANE, DIMETHICONE, TRIMETHYLSILOXYSILICATE, CYCLOPENTASILOXANE, DISTEARDIMONIUM HECTORITE, PROPYLENE CARBONATE, PHENOXYETHANOL, FRAGRANCE, CAPRYLYL GLYCOL, CHLORPHENESIN, ALPHA-ISOMETHYL IONONE, d-LIMONENE, LINALOOL, AMYL CINNAMAL, CITRONELLOL.
MAY CONTAIN :
IRON OXIDES, MANGANESE VIOLET, TITANIUM DIOXIDE, RED 7 LAKE, RED 40 LAKE, MICA, RED 27 LAKE, YELLOW 5 LAKE, RED 21 LAKE, BLUE 1 LAKE, TALC.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (FRESA TENTACIÓN) - RED
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (CAFÉ MISTERIO) - BROWN
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (ROSA DESEO) - BROWN
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (DURAZNO AMOR) - BROWN
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (ROSA GOLOSA) - PINK
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (CORAL SEDUCTOR) - PINK
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (CEREZA SEXY) - PINK
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (FUCSIA SENSUAL) - PINK
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (ROJO PASION) - RED
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (VINO CALIENTE) - PURPLE
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (PIMIENTA CALIENTE) - RED
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (CAFÉ PARISO) - BROWN
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (ROSE BLOOM) - RED
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (FRESA ENERGY) - RED
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (BURGUNDY VITAL) - RED
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (VINO SUBLIME) - RED
- PRINCIPAL DISPLAY PANEL - 2,2 g Tube Box - (ROJO RADIANT) - RED
-
INGREDIENTS AND APPEARANCE
ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (FRESA TENTACION) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-822 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-822-02 1 in 1 BOX 08/28/2015 1 NDC:14783-822-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (CAFE MISTERIO) - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-823 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-823-02 1 in 1 BOX 08/28/2015 1 NDC:14783-823-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (ROSA DESEO) - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-824 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-824-02 1 in 1 BOX 08/28/2015 1 NDC:14783-824-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (DURAZNO AMOR) - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-825 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-825-02 1 in 1 BOX 08/28/2015 1 NDC:14783-825-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS -(ROSA GOLOSA) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-826 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-826-02 1 in 1 BOX 08/28/2015 1 NDC:14783-826-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (CORAL SEDUCTOR) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-827 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-827-02 1 in 1 BOX 08/28/2015 1 NDC:14783-827-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (CEREZA SEXY) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-828 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-828-02 1 in 1 BOX 08/28/2015 1 NDC:14783-828-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (FUCSIA SENSUAL) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-829 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-829-02 1 in 1 BOX 08/28/2015 1 NDC:14783-829-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (ROJO PASION) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-830 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-830-02 1 in 1 BOX 08/28/2015 1 NDC:14783-830-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (VINO CALIENTE) - PURPLE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-831 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-831-02 1 in 1 BOX 08/28/2015 1 NDC:14783-831-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (PIMIENTA CALIENTE) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-832 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-832-02 1 in 1 BOX 08/28/2015 1 NDC:14783-832-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (CAFE PARISO) - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-113 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-113-02 1 in 1 BOX 08/28/2015 1 NDC:14783-113-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (ROSE BLOOM) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-114 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-114-02 1 in 1 BOX 08/28/2015 1 NDC:14783-114-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (FRESA ENERGY) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-115 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-115-02 1 in 1 BOX 08/28/2015 1 NDC:14783-115-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (BURGUNDY VITAL) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-116 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-116-02 1 in 1 BOX 08/28/2015 1 NDC:14783-116-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (VINO SUBLIME) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-117 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-117-02 1 in 1 BOX 08/28/2015 1 NDC:14783-117-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 ESIKA PRO PERFECT MATCH VOLUMEN SPF 20 PLUS LIP LINER FOR MAXIMUM FULLNESS - (ROJO RADIANT) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) medium-chain triglycerides (UNII: C9H2L21V7U) tridecyl stearate (UNII: A8OE252M6L) high density polyethylene (UNII: UG00KM4WR7) carnauba wax (UNII: R12CBM0EIZ) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) shea butter (UNII: K49155WL9Y) silica dimethyl silylate (UNII: EU2PSP0G0W) phenoxyethanol (UNII: HIE492ZZ3T) cetearyl ethylhexanoate (UNII: 9M64UO4C25) glycerin (UNII: PDC6A3C0OX) caprylyl glycol (UNII: 00YIU5438U) chlorphenesin (UNII: I670DAL4SZ) ceramide np (UNII: 4370DF050B) saccharin sodium (UNII: SB8ZUX40TY) sorbitan isostearate (UNII: 01S2G2C1E4) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) sucrose cocoate (UNII: 3H18P0UK73) palmitoyl lysyldioxymethionyllysine (UNII: T7A529FB8O) ferric oxide red (UNII: 1K09F3G675) manganese violet (UNII: 72M48QQV8Q) mica (UNII: V8A1AW0880) d&c red no. 30 (UNII: 2S42T2808B) aluminum oxide (UNII: LMI26O6933) titanium dioxide (UNII: 15FIX9V2JP) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) d&c red no. 7 (UNII: ECW0LZ41X8) d&c red no. 21 (UNII: 08744Z6JNY) talc (UNII: 7SEV7J4R1U) fd&c blue no. 1 (UNII: H3R47K3TBD) Isododecane (UNII: A8289P68Y2) dimethicone (UNII: 92RU3N3Y1O) cyclomethicone 5 (UNII: 0THT5PCI0R) disteardimonium hectorite (UNII: X687XDK09L) propylene carbonate (UNII: 8D08K3S51E) fd&c red no. 40 (UNII: WZB9127XOA) isomethyl-.alpha.-ionone (UNII: 9XP4LC555B) limonene, (+)- (UNII: GFD7C86Q1W) linalool, (+/-)- (UNII: D81QY6I88E) .alpha.-amylcinnamaldehyde (UNII: WC51CA3418) .beta.-citronellol, (r)- (UNII: P01OUT964K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-118-02 1 in 1 BOX 08/28/2015 1 NDC:14783-118-01 2.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/28/2015 Labeler - Ventura International LTD (603192787) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(14783-822, 14783-823, 14783-824, 14783-825, 14783-826, 14783-827, 14783-828, 14783-829, 14783-830, 14783-831, 14783-832, 14783-822, 14783-113, 14783-114, 14783-115, 14783-116, 14783-117, 14783-118)