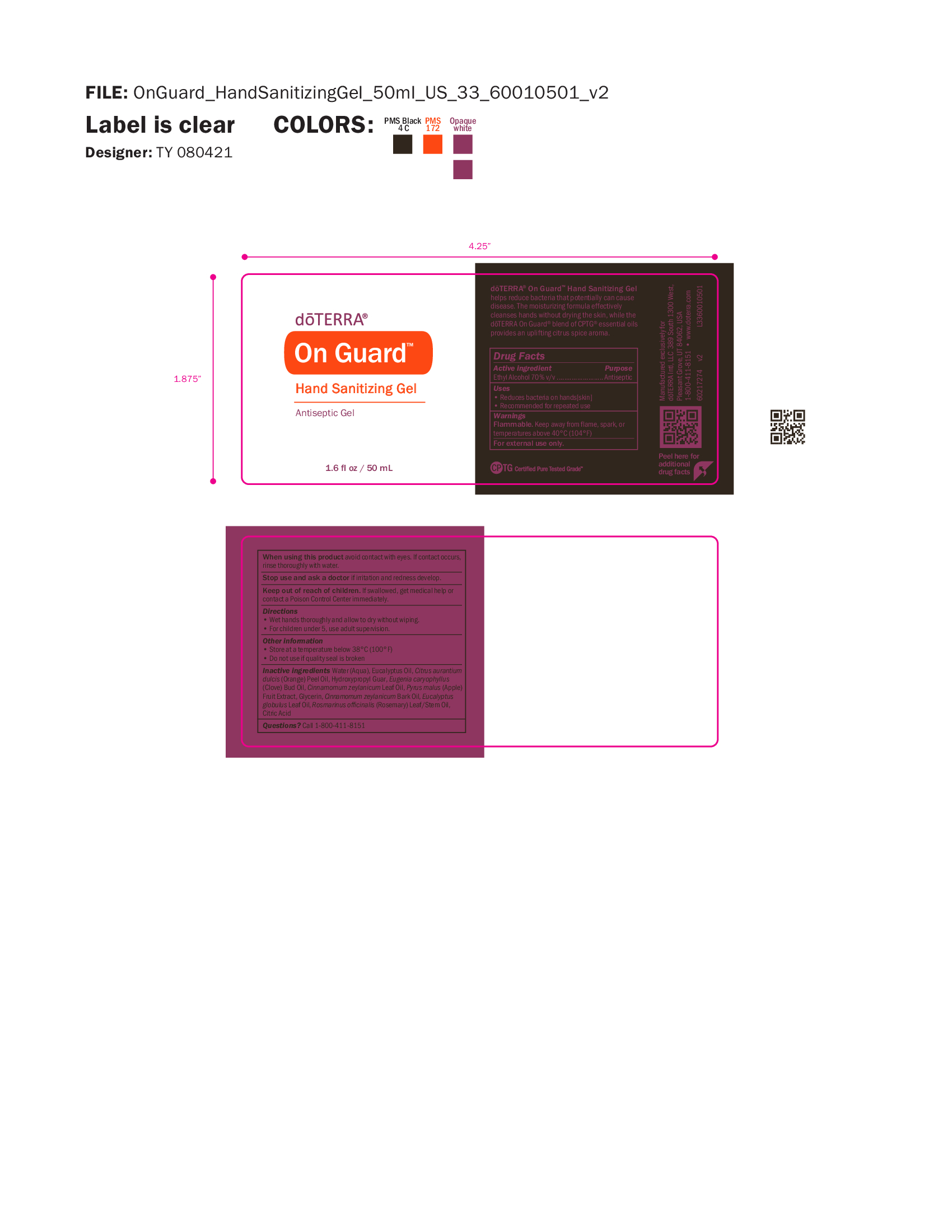
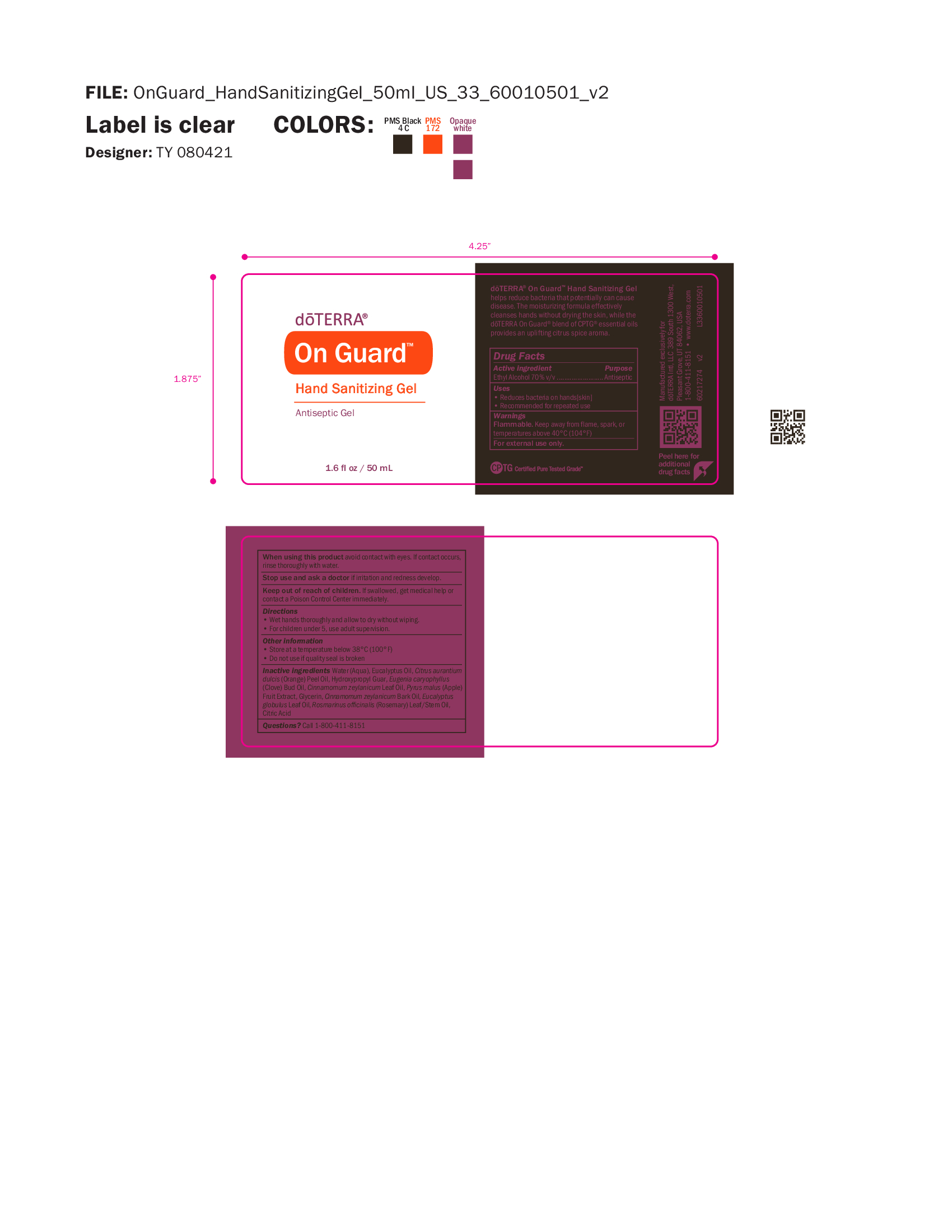
Label: ON GUARD SANITIZING GEL- alcohol gel
- NDC Code(s): 71630-098-50
- Packager: doTERRA International, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Antiseptic, Hand Sanitizer
- Uses
- Warnings
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients Water (Aqua), Eucalyptus Oil, Citrus aurantium
dulcis (Orange) Peel Oil, Hydroxypropyl Guar, Eugenia caryophyllus
(Clove) Bud Oil, Cinnamomum zeylanicum Leaf Oil, Pyrus malus (Apple)
Fruit Extract, Glycerin, Cinnamomum zeylanicum Bark Oil, Eucalyptus
globulus Leaf Oil, Rosmarinus officinalis (Rosemary) Leaf/Stem Oil,
Citric Acid - QUESTIONS
- Primary Label
-
INGREDIENTS AND APPEARANCE
ON GUARD SANITIZING GEL
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71630-098 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EUCALYPTUS OIL (UNII: 2R04ONI662) ORANGE OIL (UNII: AKN3KSD11B) HYDROXYPROPYL GUAR (2500-4500 MPA.S AT 1%) (UNII: 3A1I7376TC) CLOVE OIL (UNII: 578389D6D0) CINNAMON LEAF OIL (UNII: S92U8SQ71V) GLYCERIN (UNII: PDC6A3C0OX) CINNAMON BARK OIL (UNII: XE54U569EC) HYDRATED SILICA (UNII: Y6O7T4G8P9) BORON (UNII: N9E3X5056Q) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) ROSEMARY OIL (UNII: 8LGU7VM393) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71630-098-50 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/15/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 08/15/2022 Labeler - doTERRA International, LLC (832274935) Establishment Name Address ID/FEI Business Operations Universal Packaging Systems Inc (DBA PakLabs) 177711082 manufacture(71630-098)

 Primary Label
Primary Label