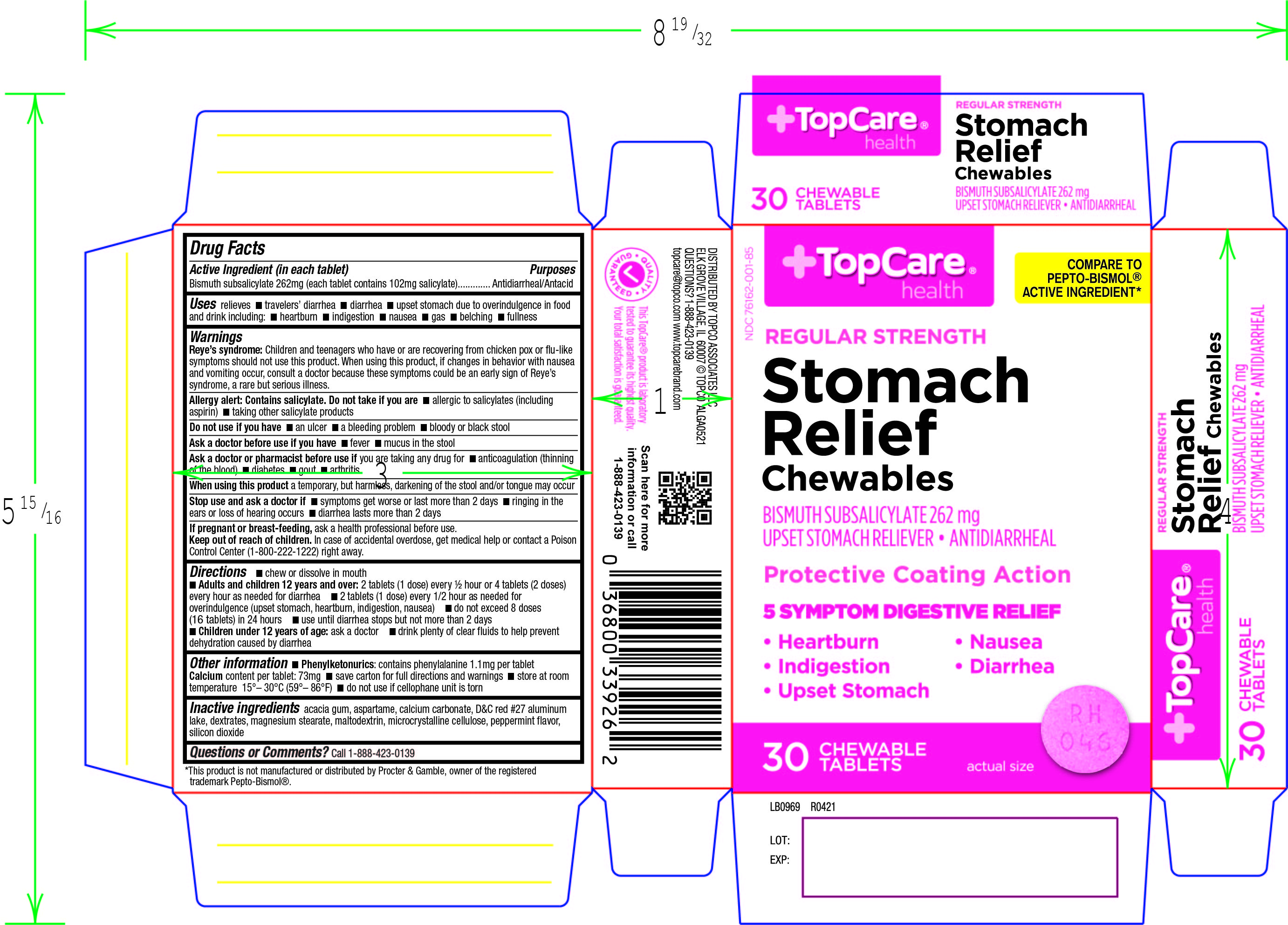
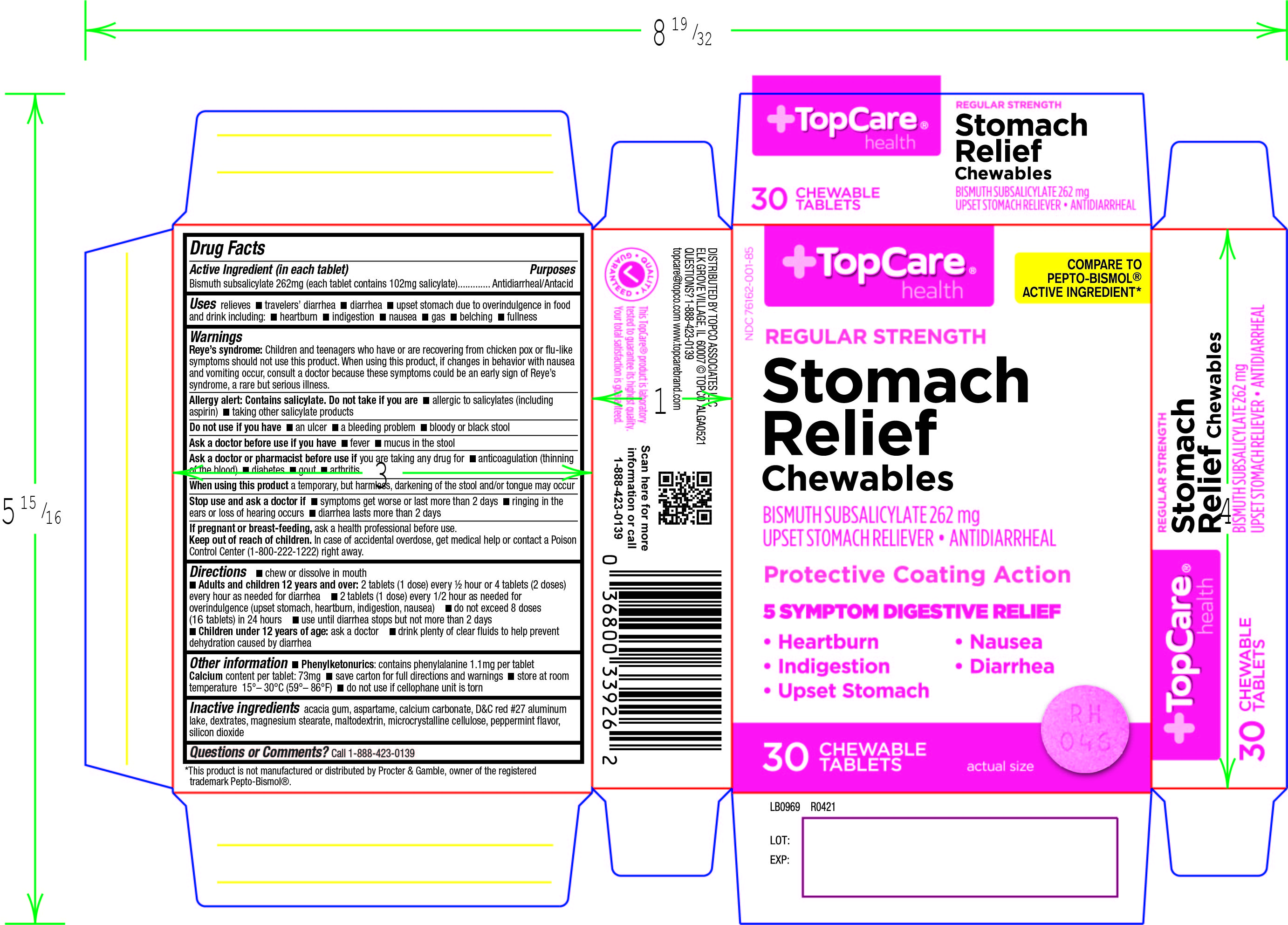
Label: TOPCARE STOMACH RELIEF ORIGINAL STRENGTH- bismuth subsalicylate tablet
- NDC Code(s): 76162-001-85
- Packager: TopCo Associates, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 14, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Reye’s syndrome
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert
Contains salicylate. Do not take if you are
- allergic to salicylates (including aspirin)
- taking other salicylate products
Ask a doctor or pharmacist before use if
you are taking any drug for
- anticoagulation (thinning of the blood)
- diabetes
- gout
- arthritis
- Keep out of reach of children
-
Directions
- chew or dissolve in mouth
Adults and children 12 years and over: 2 tablets every ½ to 1 hour as needed
- do not exceed 16 tablets in 24 hours
- use until diarrhea stops but not more than 2 days
Children under 12 years of age: ask a doctor
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- Other information
- Inactive ingredients
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TOPCARE STOMACH RELIEF ORIGINAL STRENGTH
bismuth subsalicylate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76162-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISMUTH SUBSALICYLATE (UNII: 62TEY51RR1) (BISMUTH CATION - UNII:ZS9CD1I8YE) BISMUTH SUBSALICYLATE 262 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) ASPARTAME (UNII: Z0H242BBR1) CALCIUM CARBONATE (UNII: H0G9379FGK) D&C RED NO. 27 (UNII: 2LRS185U6K) DEXTRATES (UNII: G263MI44RU) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PEPPERMINT (UNII: V95R5KMY2B) Product Characteristics Color pink Score no score Shape ROUND Size 17mm Flavor Imprint Code RH046 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76162-001-85 6 in 1 CARTON 12/23/2014 1 5 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M008 12/23/2014 Labeler - TopCo Associates, LLC (006935977)