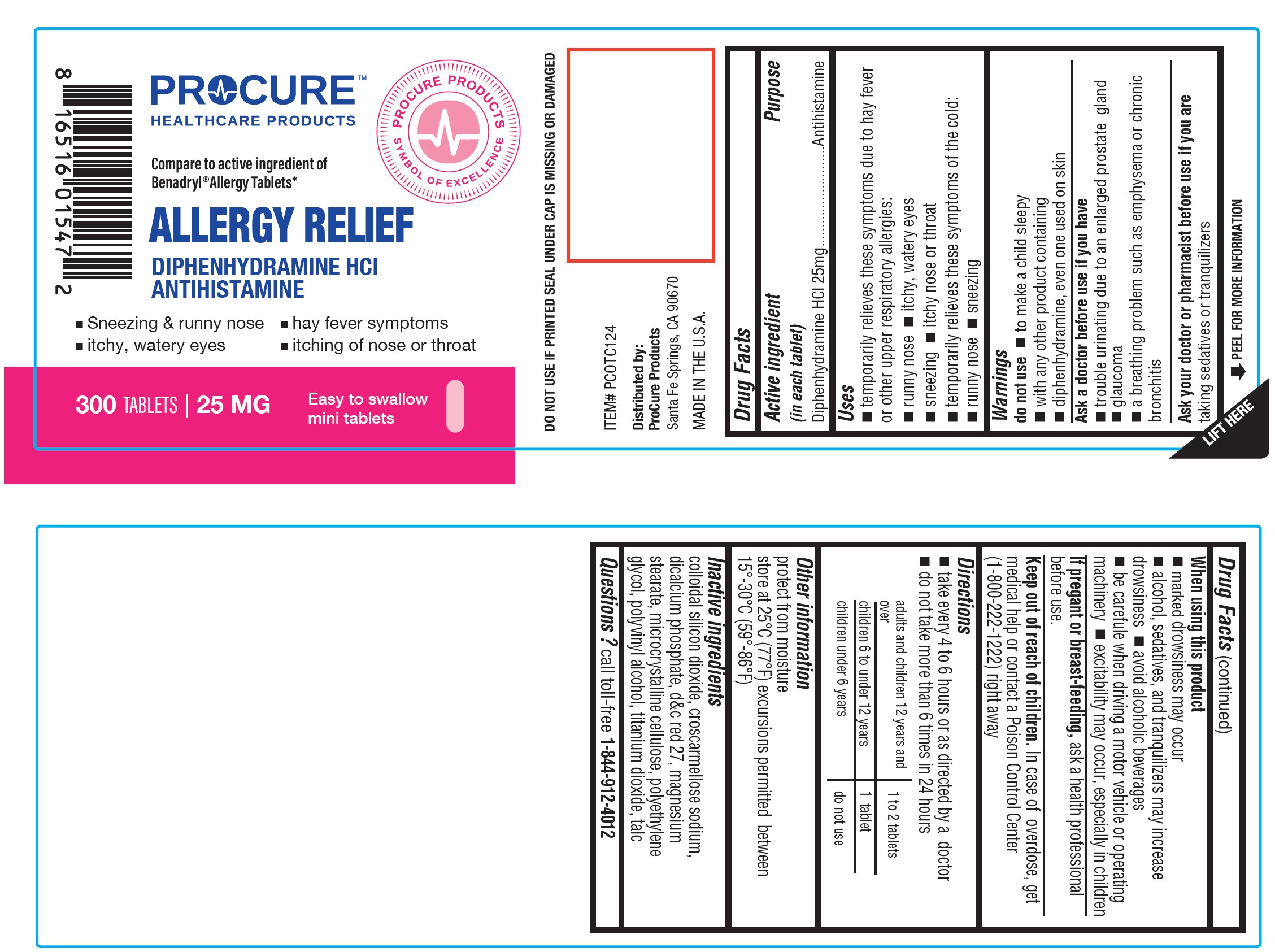
Label: ALLERGY RELIEF- diphenhydramine hydrochloride tablet
- NDC Code(s): 55681-322-03
- Packager: TWIN MED LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if you have
- trouble urinating due to an enlarged prostate gland
- glaucoma ? a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if youaretaking sedatives or tranquilizers
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- excitability may occur, especially in children ? alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- If pregnant or breast-feeding;
- Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
diphenhydramine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55681-322 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 27 (UNII: 2LRS185U6K) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYVINYL ALCOHOL (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score no score Shape CAPSULE Size 11mm Flavor Imprint Code I3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55681-322-03 300 in 1 BOTTLE; Type 0: Not a Combination Product 07/15/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/15/2022 Labeler - TWIN MED LLC (009579330)