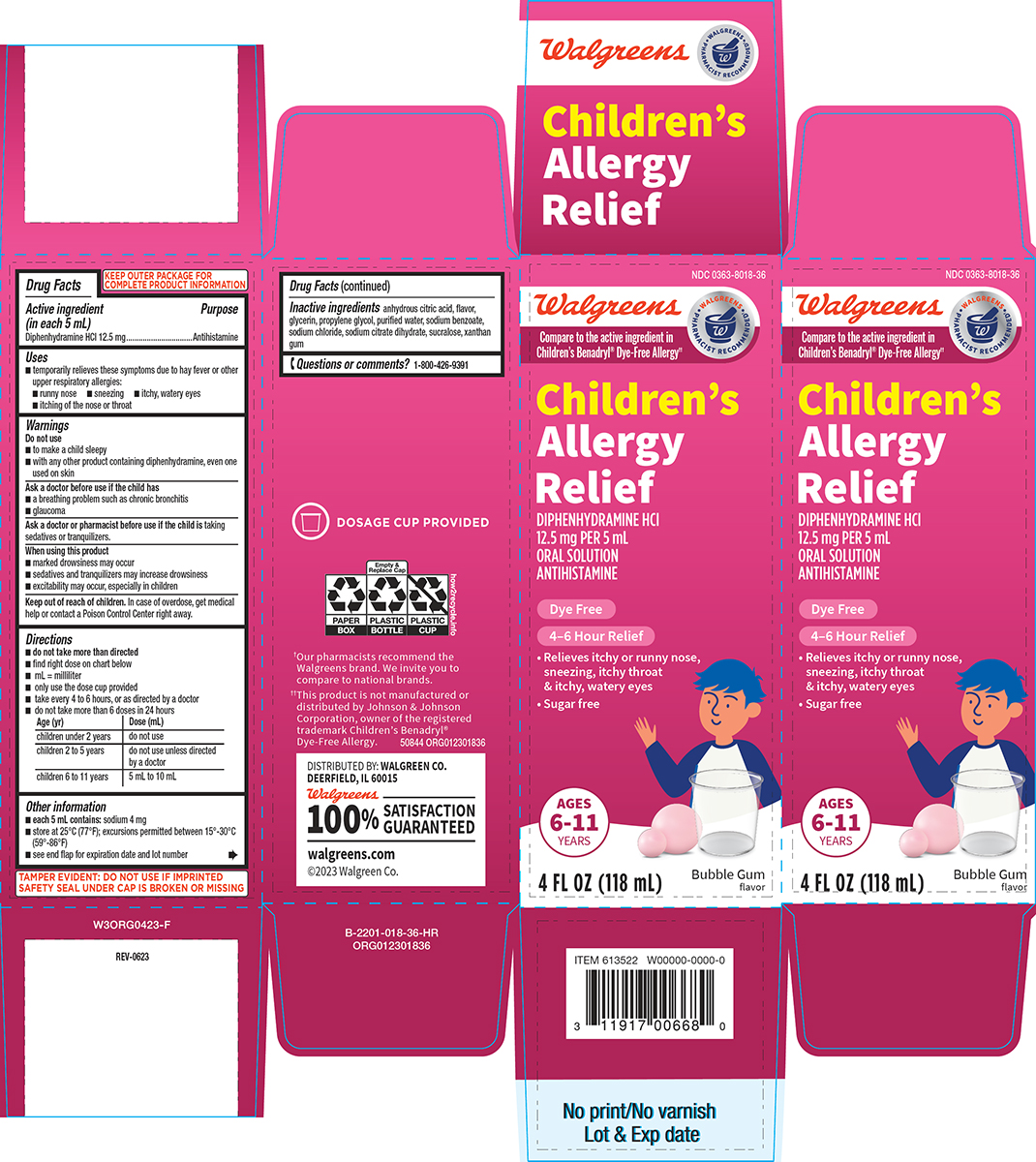
Label: CHILDRENS ALLERGY RELIEF- diphenhydramine hcl solution
- NDC Code(s): 0363-8018-36
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 5 mL)
- Purpose
- Uses
- Warnings
-
Directions
- do not take more than directed
- find right dose on chart below
- mL = milliliter
- only use the dose cup provided
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 doses in 24 hours
Age (yr) Dose (mL) children under 2 years do not use children 2 to 5 years do not use unless directed by a doctor children 6 to 11 years 5 mL to 10 mL - Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
NDC 0363-8018-36
Walgreens
WALGREENS PHARMACIST RECOMMENDED†
Compare to the active ingredient in
Children’s Benadryl® Dye-Free Allergy††Children’s
Allergy
ReliefDIPHENHYDRAMINE HCl
12.5 mg PER 5 mL
ORAL SOLUTION
ANTIHISTAMINEDye Free
4–6 Hour Relief
• Relieves itchy or runny nose,
sneezing, itchy throat
& itchy, watery eyes
• Sugar freeAGES
6-11
YEARS4 FL OZ (118 mL)
Bubble Gum
flavorTAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSINGDOSAGE CUP PROVIDED
†Our pharmacists recommend the
Walgreens brand. We invite you to
compare to national brands.††This product is not manufactured or
distributed by Johnson & Johnson
Corporation, owner of the registered
trademark Children’s Benadryl®
Dye-Free Allergy. 50844 ORG012301836DISTRIBUTED BY: WALGREEN CO.
DEERFIELD, IL 60015Walgreens
100% SATISFACTION
GUARANTEED
walgreens.com
©2023 Walgreen Co.
Walgreens 44-018
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLERGY RELIEF
diphenhydramine hcl solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-8018 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-8018-36 1 in 1 CARTON 05/18/2023 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/18/2023 Labeler - Walgreen Company (008965063) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 manufacture(0363-8018) , pack(0363-8018)