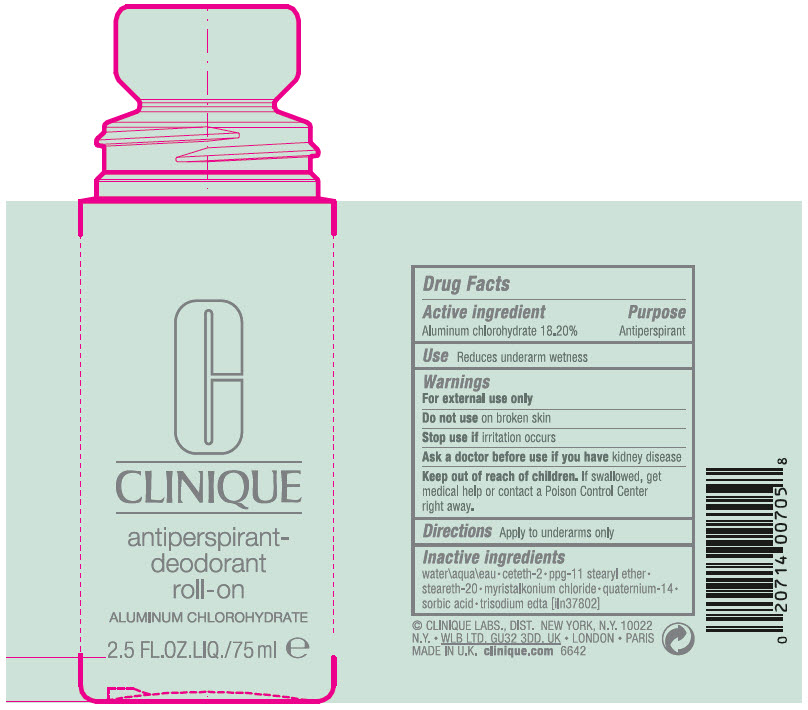
Label: ANTIPERSPIRANT-DEODORANT ROLL-ON- aluminum chlorohydrate liquid
- NDC Code(s): 49527-076-01
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 75 ml Bottle Label
-
INGREDIENTS AND APPEARANCE
ANTIPERSPIRANT-DEODORANT ROLL-ON
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-076 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 182 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETETH-2 (UNII: 7H8VAM7778) POLYPROPYLENE GLYCOL 11 STEARYL ETHER (UNII: S4G2J0Y0LG) STEARETH-20 (UNII: L0Q8IK9E08) MYRISTALKONIUM CHLORIDE (UNII: 0W255OL75T) QUATERNIUM-14 (UNII: ZGE94G6AGI) SORBIC ACID (UNII: X045WJ989B) EDETATE TRISODIUM (UNII: 420IP921MB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-076-01 75 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/15/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/15/2020 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Whitman Laboratories Ltd. 216866277 manufacture(49527-076) , pack(49527-076) , label(49527-076)