Label: T3 WOUND WASH- benzalkonium chloride solution
- NDC Code(s): 72053-004-04
- Packager: Patient Focused Telehealth
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
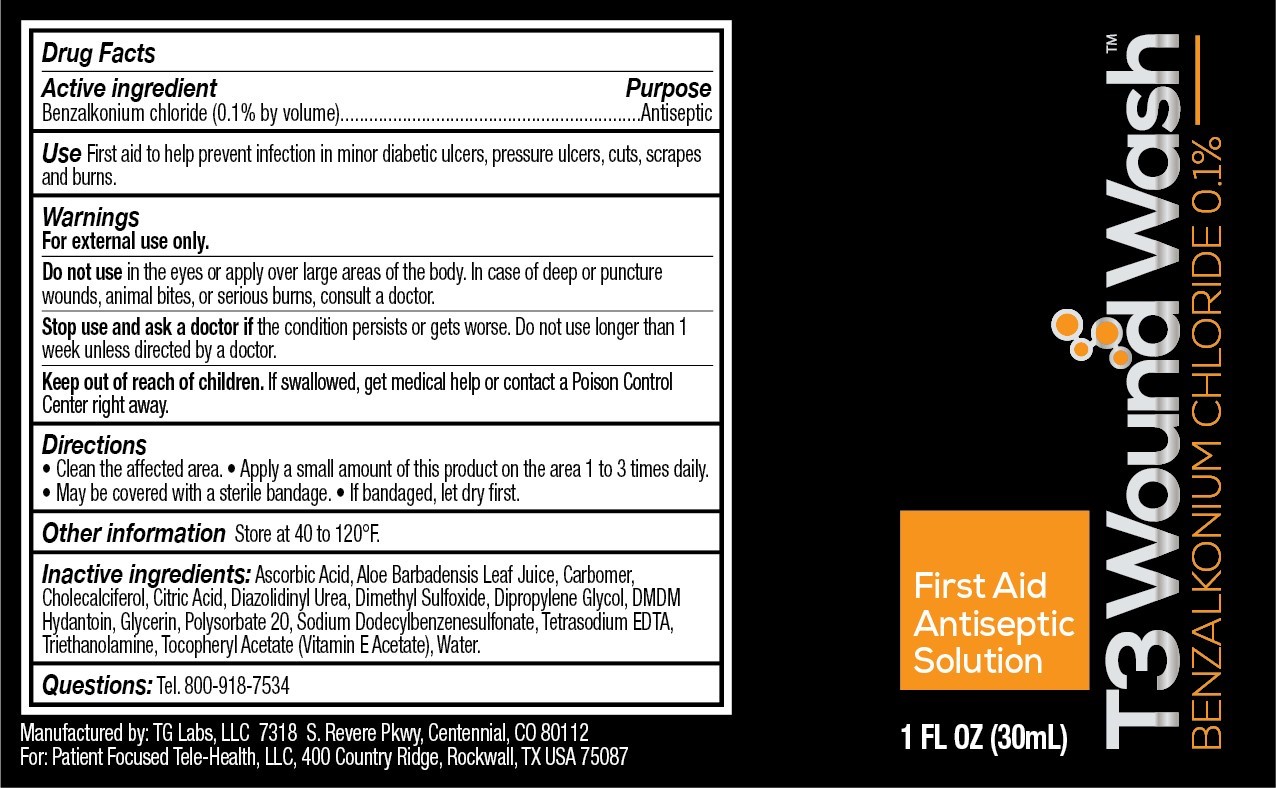
- Active Ingredient
- purpose
- Use
- Warnings
- When using this product
- stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients
- Questions
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
T3 WOUND WASH
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72053-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.03 g in 30 mL Inactive Ingredients Ingredient Name Strength DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) TROLAMINE (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CHOLECALCIFEROL (UNII: 1C6V77QF41) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) DMDM HYDANTOIN (UNII: BYR0546TOW) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM DODECYLBENZENESULFONATE (UNII: 554127163Y) ALOE VERA LEAF (UNII: ZY81Z83H0X) POLYSORBATE 20 (UNII: 7T1F30V5YH) EDETATE SODIUM (UNII: MP1J8420LU) ASCORBIC ACID (UNII: PQ6CK8PD0R) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) DIPROPYLENE GLYCOL (UNII: E107L85C40) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72053-004-04 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/25/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 07/25/2022 Labeler - Patient Focused Telehealth (081008911)

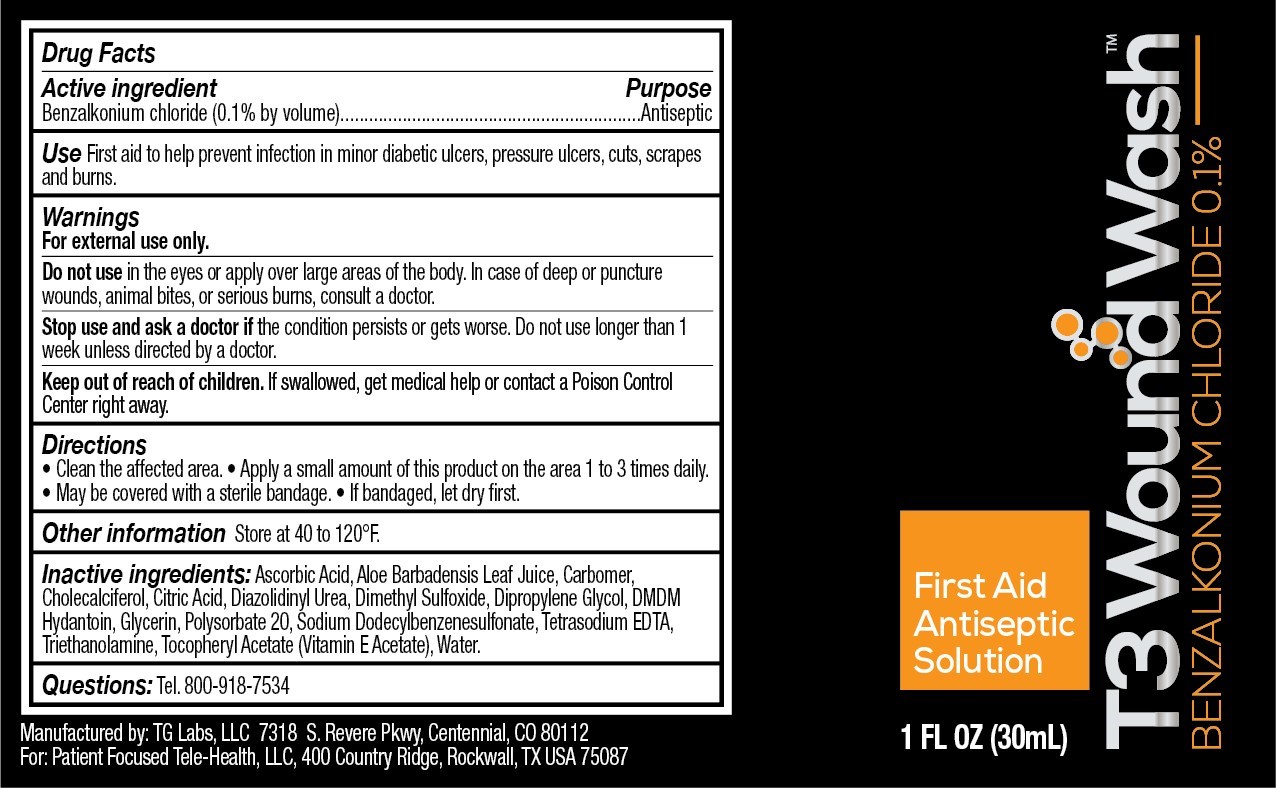
 T3 Wound Wash
T3 Wound Wash