Label: SODIUM CHLORIDE injection
- NDC Code(s): 0019-1188-27, 0019-1188-81
- Packager: Liebel-Flarsheim Company LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Sodium Chloride Injection USP 0.9% safely and effectively. See full prescribing information for Sodium Chloride Injection USP 0.9%.
Sodium Chloride Injection USP 0.9% Prefilled Syringes, For Intravascular Use Only
Initial U.S. Approval: 2006INDICATIONS AND USAGE
- For use in flushing compatible contrast agents through Liebel-Flarsheim intravenous administration sets into indwelling intravascular access devices (1)
DOSAGE AND ADMINISTRATION
- For single patient use only. (2)
- Determine the volume of flush based on the imaging procedure, location of the vascular access device, length of tubing between power injector and vascular access device, and the contrast agent package insert. (2)
- Individualize the volume of flush based on body weight, fluid status and concomitant medical conditions. (2)
- Typical flush volumes for adults are 10 to 25 mL per injection at rates not to exceed 10 mL/sec. (2)
- May be used for additional infusion to maintain the patency of vascular access at a typical infusion rate of 0.5 to 1 mL per minute. (2)
- Do not use if packaging is damaged, wet, or not intact, if syringe or its tip cap shows signs of damage, leakage or displacement. Do not use if solution is hazy, cloudy, discolored, or contains particulate matter. (2)
- Use aseptic technique. (2)
- Expel residual air from the syringe and tubing prior to connection with the patient’s vascular access. (2)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- None (4)
WARNINGS AND PRECAUTIONS
- Remove all air from the syringe and associated tubing prior to injection. (5.1)
- May cause fluid overload in patients with congestive heart failure, severe renal insufficiency, and in clinical states with edema, sodium retention, or hypernatremia. (5.3)
- Establish intravascular catheter patency prior to administration. (5.4)
ADVERSE REACTIONS
- Adverse reactions due to solution or administration technique may include: air embolism with stroke, chest pain, and dyspnea, arrhythmia, hypotension, myocardial infarction, sepsis, febrile response, local tenderness, infection at the site of injection, venous thrombosis or phlebitis extending from injection site, extravasation, fluid overload and hypervolemia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Liebel-Flarsheim Company LLC at 1-855-266-5037 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Pregnancy: It is not known whether Sodium Chloride Injection USP 0.9% can cause fetal harm. (8.1)
- Pediatrics: Safety and effectiveness have not been established in pediatric patients. (8.4)
- Geriatrics: Dosing for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. (8.5)
Revised: 9/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Drug Handling
2.2 125 mL Syringe Assembly and Inspection
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Air Embolism
5.2 Infectious Complications
5.3 Fluid Overload
5.4 Extravasation
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
16 HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
- 125 mL Syringe: Sodium Chloride Injection USP 0.9% is indicated for use in flushing compatible contrast agents through Liebel-Flarsheim intravenous administration sets into indwelling intravascular access devices only when delivered by the following Liebel-Flarsheim power injectors: Angiomat®, Illumena®, Illumena® Néo, CT9000®, CT9000® ADV, Optistat®, Optivantage® and Optistar Elite.
-
2 DOSAGE AND ADMINISTRATION
- The 125 mL syringe are for single patient use only.
- Determine the volume of the saline flush for each patient individually based, in part, on the imaging procedure, the location of the vascular access device, the length of tubing between the Liebel-Flarsheim contrast agent power injector and the vascular access device and the recommendations made on the package insert for the contrast agent. Typical Sodium Chloride Injection USP 0.9% flush volumes following contrast agent administration in adults are 10 to 25 mL per injection at rates not to exceed 10 mL/sec.
- Use of some Liebel-Flarsheim contrast agent power injectors allows for additional infusion of Sodium Chloride Injection USP 0.9% to maintain the patency of vascular access. Typical infusion rates used for this purpose are in the range of 0.5 to 1 mL per minute.
- Individualize infusion rates and flush volumes for each patient based on their body weight, fluid status and concomitant medical conditions.
- Consult the Liebel-Flarsheim contrast agent power injector manual for proper use.
2.1 Drug Handling
- Inspect the syringe for signs of break in sterility.
- Do not use if the syringe or its tip cap shows signs of damage, leakage or displacement.
- Do not use if the solution is hazy, cloudy, discolored or contains particulate matter.
- Use aseptic technique.
- Expel residual air in both the syringe and tubing prior to connection with the patient’s vascular access.
- Instructions for assembly and inspection of the Sodium Chloride Injection USP 0.9% syringes prior to use are printed on this sheet.
2.2 125 mL Syringe Assembly and Inspection
NOTE: Exterior of syringe is not sterile.
Contents of syringe and area under tip cap and piston ribs are sterile and should be treated accordingly.
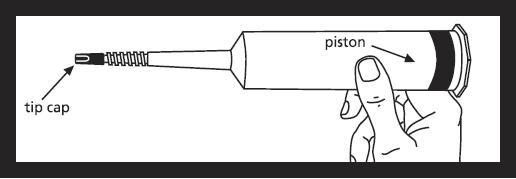
Remove syringe from carton and inspect the area around the tip cap and outside of piston for signs of leakage. Do not use if leakage is observed. Load syringe into power injector.
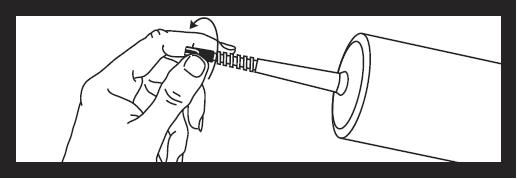
To remove tip cap from syringe, push in and twist off, then discard.
The area under the cap is sterile. Caution should now be used when handling.
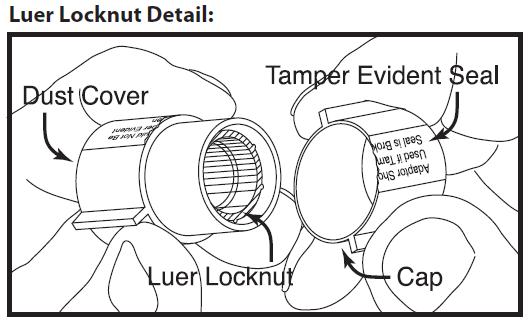
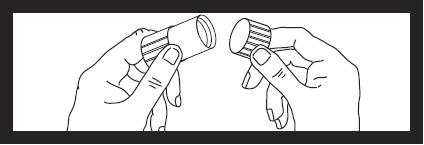
Next remove cap from luer locknut dust cover by twisting to break tamper evident seal. Discard cap.
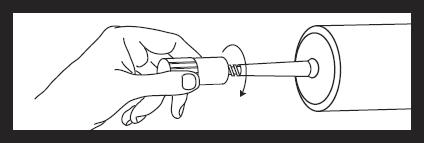
Attach luer locknut to syringe by holding dust cover and screwing to the stop.
Remove and discard dust cover when ready to attach sterile connector tubing.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Air Embolism
Remove all air from the syringe and associated tubing prior to injection to avoid air embolus with the associated risk of stroke, organ ischemia and/or infarction, and death.
5.2 Infectious Complications
Use of a damaged syringe or failure to maintain aseptic technique may result in infection, sepsis and death [see Dosage and Administration (2)].
5.3 Fluid Overload
Sodium Chloride Injection USP 0.9% may cause fluid overload in patients with congestive heart failure, severe renal insufficiency, and in clinical states with edema, sodium retention, or hypernatremia.
Consider each patient’s age, body weight, fluid status, concomitant medical conditions and planned radiological procedure to determine if use of Sodium Chloride Injection USP 0.9% is appropriate.
5.4 Extravasation
Extravasation of the Sodium Chloride Injection USP 0.9% may cause mechanical compression of neurovascular structures. Extravasation of contrast agent may result in tissue injury by osmolar and direct cytotoxicity (see package inserts of specific contrast agents). Establish intravascular catheter patency prior to the administration of Sodium Chloride Injection USP 0.9%.
-
6 ADVERSE REACTIONS
Reported adverse reactions include:
- air embolization with stroke, chest pain, and dyspnea
- arrhythmia
- hypotension
- myocardial infarction
- sepsis
- febrile response
- local tenderness
- infection at the site of injection
- venous thrombosis or phlebitis extending from injection site
- extravasation
- fluid overload
- hypervolemia
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Administration of Sodium Chloride Injection USP 0.9% is not known to cause major birth defects, miscarriage or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with Sodium Chloride Injection USP 0.9.%All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
Appropriate administration of Sodium Chloride Injection USP 0.9% is not known to cause harm to a breastfed infant.8.4 Pediatric Use
Safety and effectiveness of Sodium Chloride Injection USP 0.9% administered by power injection in pediatric patients have not been established. Administration of Sodium Chloride Injection USP 0.9% to pediatric patients by power injection is not recommended. When performing manual injection of Sodium Chloride Injection USP 0.9% to pediatric patients, take into account the patient’s weight, fluid status, and concomitant medical conditions to determine if use of Sodium Chloride Injection USP 0.9% is appropriate.
The safety of manual injection of Sodium Chloride Injection USP 0.9% in pediatric patients is supported by reported clinical experience with intravenous infusion and flush of sodium chloride injection in pediatric patients.
To minimize the risk of fluid overload, use the smallest dose of Sodium Chloride Injection USP 0.9% necessary for manually flushing contrast agent through the vascular access line.
8.5 Geriatric Use
No clinical studies of Sodium Chloride Injection USP 0.9% were conducted. Other reported clinical experience with sodium chloride injection has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
Use of Sodium Chloride Injection USP 0.9% may pose a threat of overdose marked by electrolyte disturbance and/or fluid overload, particularly in pediatric patients and patients with compromised renal or cardiac function. In the event of overdosage, discontinue the infusion, reevaluate the patient and institute appropriate corrective action.
-
11 DESCRIPTION
Sodium Chloride Injection USP 0.9% is a formulation of sodium chloride in Water for Intravascular Injection. No preservative, antimicrobial agent or buffer is added. Sodium Chloride Injection USP 0.9% is provided as a sterile, nonpyrogenic, clear, colorless, odorless solution.
Molecular formula = NaCl MW = 58.44
Each mL of Sodium Chloride Injection USP 0.9% contains 9 mg of sodium chloride. The pH is 4.5 to 7.0. The osmolarity is 308 mOsm/L (calc.).
Sodium Chloride Injection USP 0.9% is provided in a 125 mL syringe with a 125 mL fill. The syringes are for single patient use and are disposable and not meant for reuse.
- 12 CLINICAL PHARMACOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Sodium Chloride Injection USP 0.9% is a clear, colorless, odorless solution containing 9 mg/mL of sodium chloride. Sodium Chloride Injection USP 0.9% is supplied in 125 mL prefilled syringes containing 125 mL of solution. Each syringe is sealed with rubber closures and the contents are sterile. The 125 mL syringe is supplied with a luer locknut adapter which is cleared for manufacture and distribution as a device under 510(K) number 862653. The syringes are contained in shipping cartons with the following configurations:
Volume Packaging NDC 125 mL
in plastic syringes20 syringes per carton 0019-1188-81 125 mL
in plastic RFID-Tagged Syringes*20 syringes per carton 0019-1188-27 *Radio Frequency Identification (RFID) Technology RFID-Tagged Syringe Description
This information is for Ultraject® syringes containing Sodium Chloride Injection USP 0.9% that has been labeled with a Radio Frequency Identification (RFID) tag. When used with an RFID-enabled Optivantage Injector, this tag allows for the exchange of product information such as lot number, expiration, concentration, and identification of the syringe as being unused prior to use and used after product administration. Patient information is not utilized in any form with this RFID technology. Sodium Chloride Injection USP 0.9% product quality is not impacted with the use of this RFID tag. Sodium Chloride Injection USP 0.9% RFID syringes require no special handling and should be stored at the conditions listed for the drug product.
RFID-Tagged Syringe Directions for Use
For the RFID technology to function, the syringe must be used with an Optivantage Injector with RFID technology. Function of the RFID technology is not dependent on syringe orientation as it is placed in the injector. Instructions for use of the injector are provided on the injector interface screens and operator’s manual.
If the RFID tag is damaged or otherwise non-functional, the injector will notify the user. Should this occur, the Sodium Chloride Injection USP 0.9% syringe with the non-functional RFID tag may still be used but no data will be transferred to the injector.
Regarding interference with medical devices, the RFID tag and injector system meet the IEC 60601-1-2 requirements for emission and immunity standards for medical devices. Follow all manufacturers’ guidelines and do not operate any part of the Optivantage Injector System and RFID-tagged syringes within 6 inches (15 cm) of a pacemaker and/or defibrillator.
Storage
Store Sodium Chloride Injection USP 0.9% syringes and RFID-tagged syringes at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
PROTECT FROM FREEZING
Manufactured by:
Liebel-Flarsheim Company LLC
Raleigh, NC 27616Made in USA
GBT 11880919
Issued: 9/19
Guerbet
-
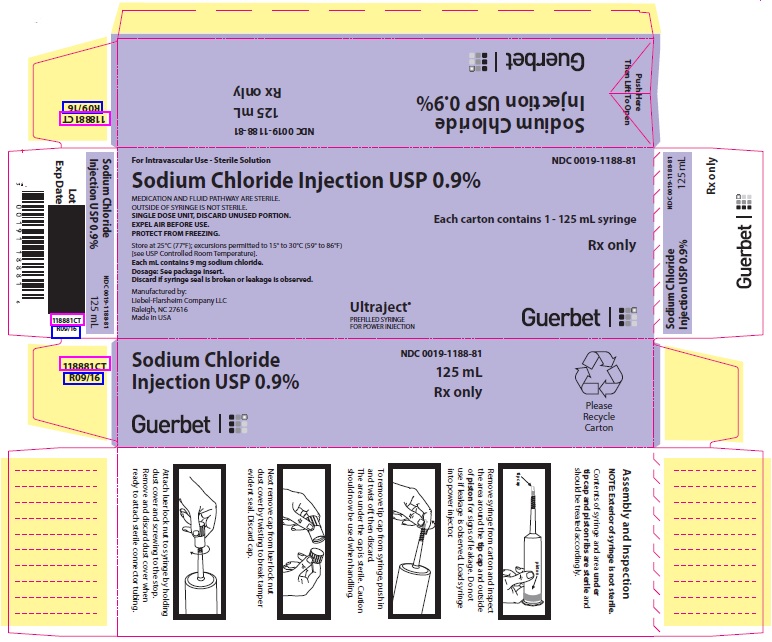
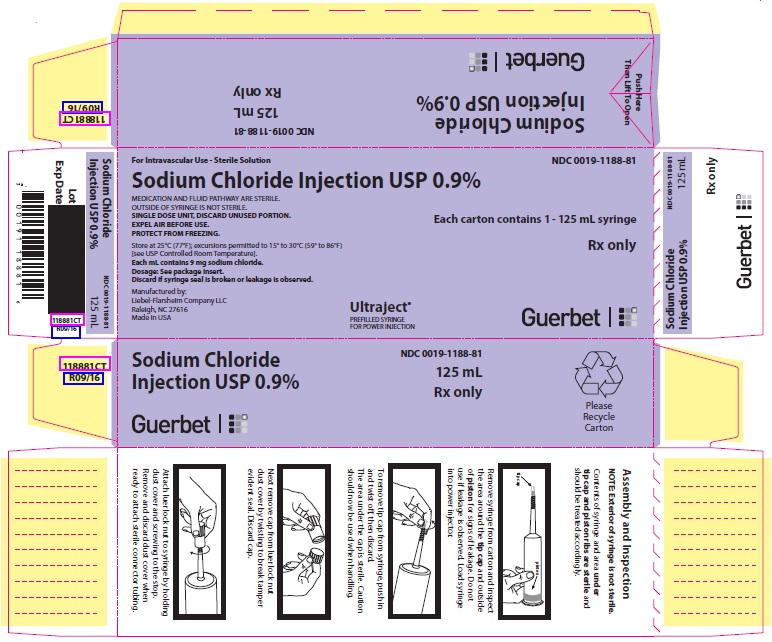
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 125 mL Syringe
For Intravascular Use
Sterile Solution
NDC 0019-1188-81
125 mL
Sodium Chloride Injection USP 0.9%
MEDICATION AND FLUID PATHWAY ARE STERILE.
OUTSIDE OF SYRINGE IS NOT STERILE.SINGLE DOSE UNIT, DISCARD UNUSED PORTION.
EXPEL AIR BEFORE USE.
PROTECT FROM FREEZING.Rx only
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Each mL contains 9 mg sodium chloride.
Dosage: See package insert.
Discard if syringe seal is broken or leakage is observed.Manufactured by:Liebel-Flarsheim Company LLC
Raleigh, NC 27616
Made in USAGuerbet
12070916

-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
sodium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0019-1188 Route of Administration INTRAVASCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE .9 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0019-1188-81 20 in 1 CARTON 09/01/2012 1 1 in 1 BOX 1 125 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:0019-1188-27 20 in 1 CARTON 09/01/2012 2 1 in 1 BOX 2 125 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021569 09/01/2012 Labeler - Liebel-Flarsheim Company LLC (057880002) Establishment Name Address ID/FEI Business Operations Avantor Performance Materials, Inc. 152791026 API MANUFACTURE(0019-1188) Establishment Name Address ID/FEI Business Operations LIEBEL-FLARSHEIM COMPANY LLC 109024984 ANALYSIS(0019-1188) , MANUFACTURE(0019-1188)