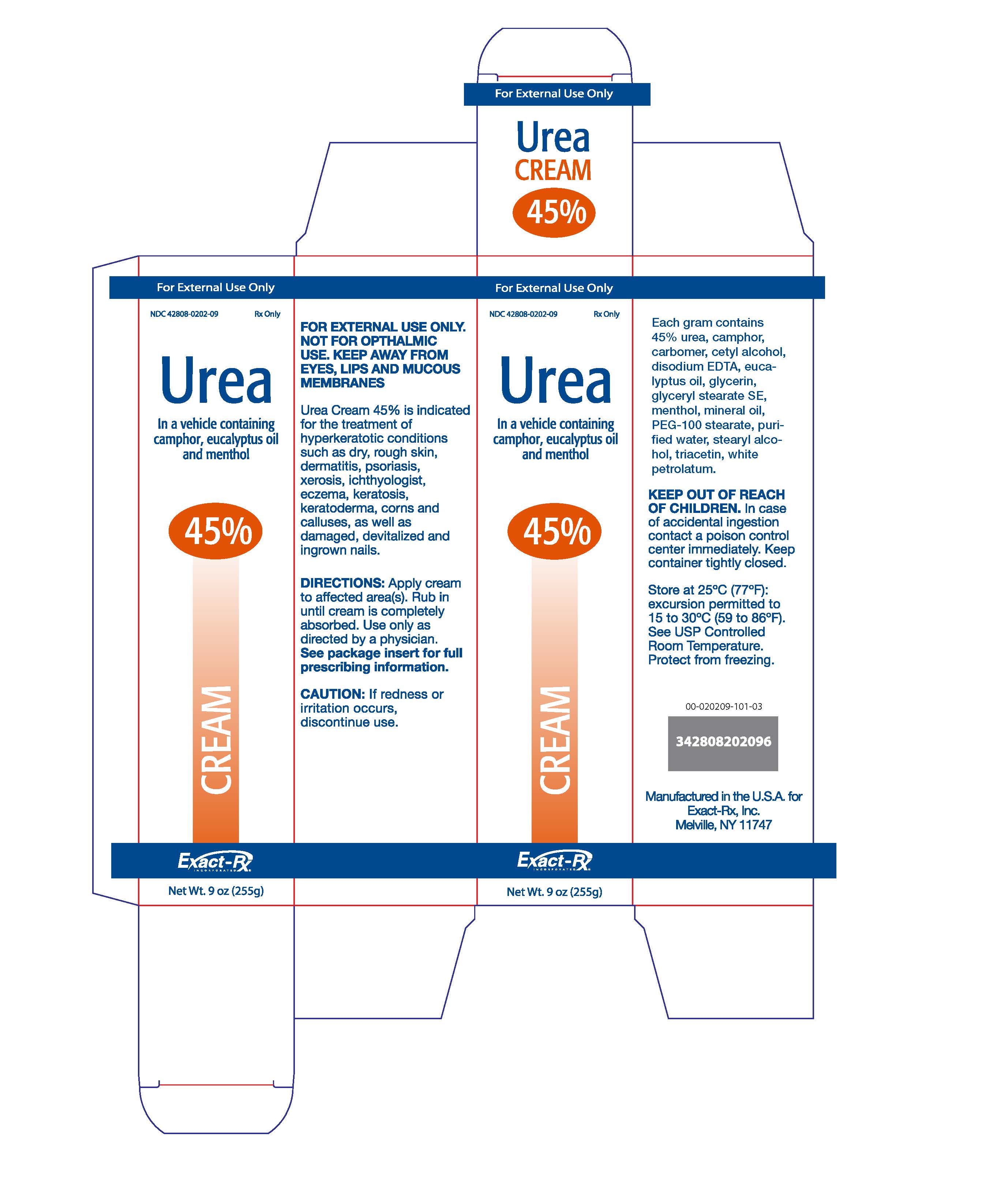
Label: UREA cream
- NDC Code(s): 42808-202-09
- Packager: Exact-Rx, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
DESCRIPTION: UREA CREAM 45%is a keratolytic emollient, which is a gentle, yet potent, tissue softener for nails and/or skin. Each gram contains 45% Urea in a cream base of: camphor, edetate disodium, alcohol, eucalyptus oil, hydroxyethyl cellulose, menthol, purified water, titanium dioxide, sodium hydroxide.
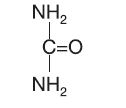
CHEMISTRY:Urea is a diamide of carbonic acid with the following chemical structure:
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY:Urea gently dissolves the intercellular matrix, which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas. Urea also hydrates and gently dissolves the intercellular matrix of the nail plate, which can result in the softening and eventual removal of devitalized nail plate tissue.
- PHARMACOKINETICS
-
INDICATIONS & USAGE
INDICATIONS AND USES:Urea 45% Cream is indicated for use in the topical treatment for debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, corns and calluses, as well as damaged devitalized and ingrown nails.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
PRECAUTIONS:Use this medication only as directed by a physician. It should not be used to treat and condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use. After applying this medication, wash hands and unaffected areas thoroughly. KEEP THIS AND ALL MEDICATION OUT OF REACH OF CHILDREN.
-
PREGNANCY
PREGNANCY:Pregnancy Category B. Animal reproduction studies have revealed no evidence of harm to the fetus, however, there are no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, Urea Cream 45% should be given to a pregnant woman only if clearly needed.
- NURSING MOTHERS
- ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UREA
urea creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42808-202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UREA (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) UREA 450 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) WHITE PETROLATUM (UNII: B6E5W8RQJ4) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) EDETATE DISODIUM (UNII: 7FLD91C86K) ALCOHOL (UNII: 3K9958V90M) EUCALYPTUS OIL (UNII: 2R04ONI662) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL (UNII: L7T10EIP3A) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PEG-100 STEARATE (UNII: YD01N1999R) MINERAL OIL (UNII: T5L8T28FGP) CETYL ALCOHOL (UNII: 936JST6JCN) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42808-202-09 1 in 1 CARTON 08/01/2011 1 255 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/01/2011 Labeler - Exact-Rx, Inc. (137953498)