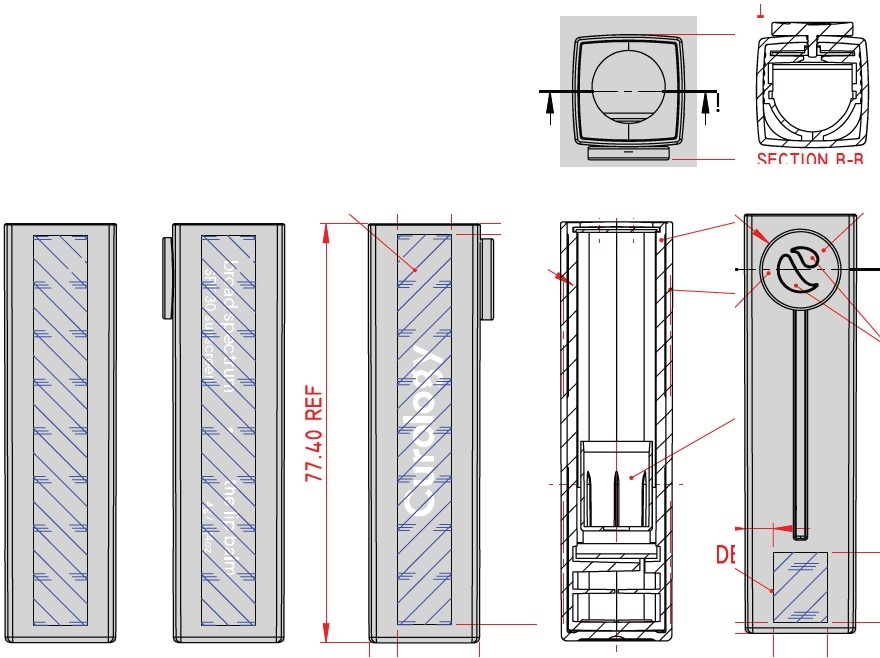
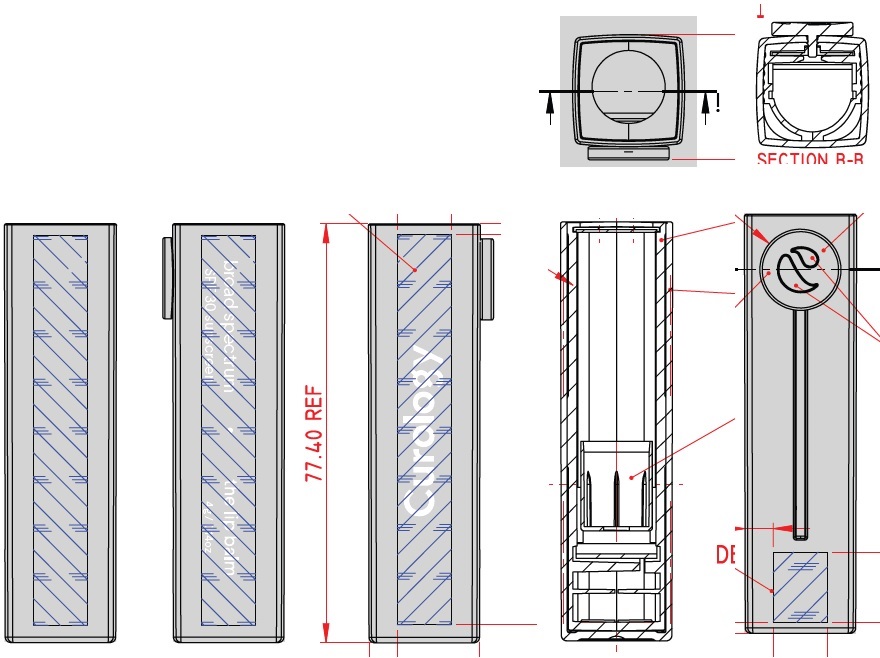
Label: CUROLOGY THE LIP BALM BROAD SPECTRUM SPF 30 SUNSCREEN- zinc oxide stick
- NDC Code(s): 82575-034-01
- Packager: Curology Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
-
Directions
- Apply generously 15 minutes before sun exposure.
- Reapply every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Children undre 6 months of age: Ask a doctor
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures.
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Other Information
-
Inactive Ingredients
Diisostearyl Dimer Dilinoleate, Synthetic Beeswax, Euphorbia Cerifera (Candelilla) Wax, Triisostearin, Helianthus Annuus (Sunflower) Seed Oil, Octyldodecyl Neopentanoate, C12-15 Alkyl Benzoate, Phytosteryl/Octyldodecyl Lauroyl Glutamate, Ozokerite, Ricinus Communis (Castor) Seed Oil, Jojoba Esters, Butyrospermum Parkii (Shea) Butter, Sucrose Tetrastearate Triacetate, Caprylic/Capric Triglyceride, Tocopherol, Tocopheryl Acetate, Caprylyl Glycol, 1,2-Hexanediol, Triethoxycaprylylsilane, Ethyl Ferulate
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
CUROLOGY THE LIP BALM BROAD SPECTRUM SPF 30 SUNSCREEN
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82575-034 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 94 mg in 1 g Inactive Ingredients Ingredient Name Strength DIISOSTEARYL DIMER DILINOLEATE (UNII: 04P17590AP) SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) CANDELILLA WAX (UNII: WL0328HX19) TRIISOSTEARIN (UNII: 71503RH8KG) SUNFLOWER OIL (UNII: 3W1JG795YI) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PHYTOSTERYL/OCTYLDODECYL LAUROYL GLUTAMATE (UNII: 65954KGO9Q) CASTOR OIL (UNII: D5340Y2I9G) SHEA BUTTER (UNII: K49155WL9Y) SUCROSE TETRASTEARATE TRIACETATE (UNII: 1K7LBQ045N) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYL FERULATE (UNII: 5B8915UELW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82575-034-01 1 in 1 CARTON 07/16/2022 1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/16/2022 Labeler - Curology Inc. (104103284)