Label: FEXOFENADINE HCL tablet
-
NDC Code(s):
69230-300-01,
69230-300-05,
69230-300-30,
69230-301-01, view more69230-301-30
- Packager: Camber Consumer Care
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 6, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT(S), in each tablet
- PURPOSE
- USE(S)
- WARNINGS
- DO NOT USE
- ASK A DOCTOR BEFORE USE IF
- WHEN USING THIS PRODUCT
- STOP USE AND ASK A DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
60 mg
adults and children 12 years of age and over
take one 60 mg tablet with water every 12 hours; do not take more than 2 tablets in 24 hours
children under 12 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
180 mg
adults and children 12 years of age and over
take one 180 mg tablet with water once a day; do not take more than 1 tablet in 24 hours
children under 12 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS
-
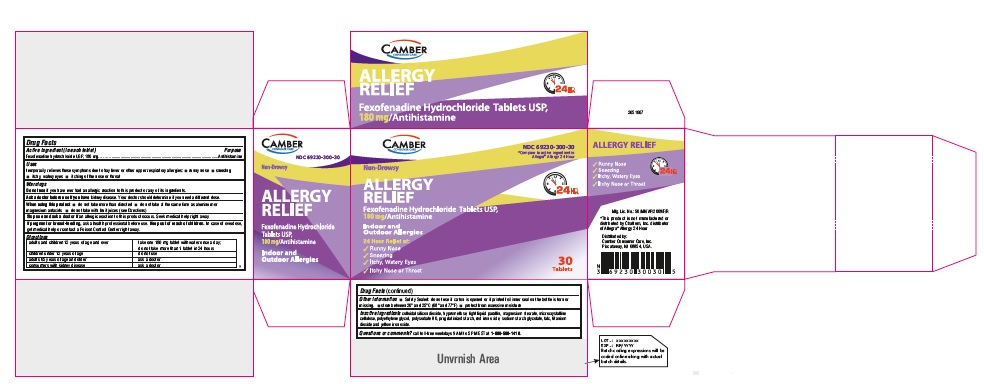
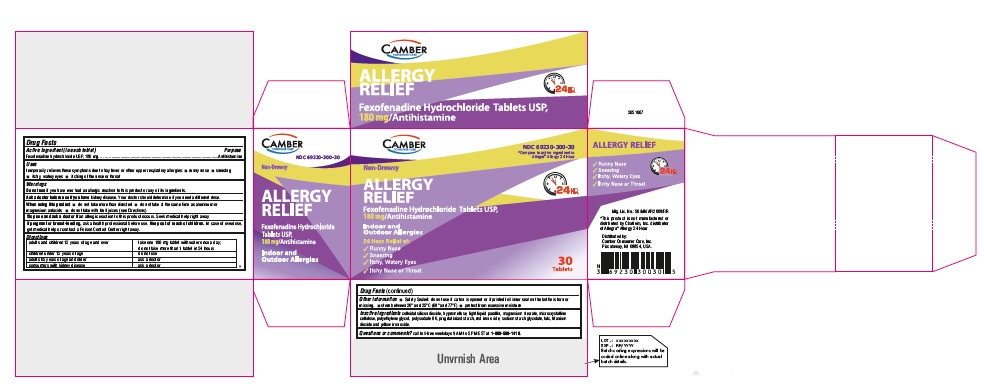
PRINCIPAL DISPLAY PANEL
Fexofenadine Hydrochloride Tablets, USP 180 mg 30s Container Label

Fexofenadine Hydrochloride Tablets, USP 180 mg 30s Carton Label
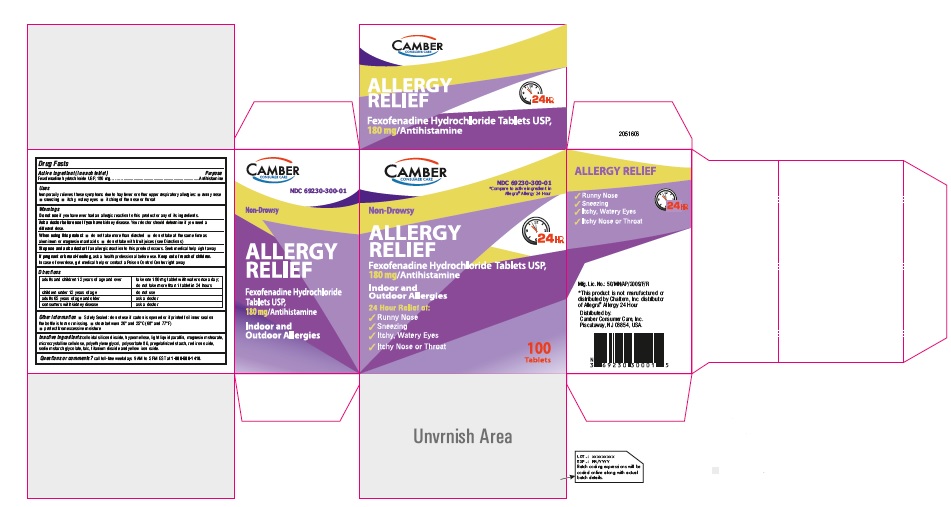
Fexofenadine Hydrochloride Tablets, USP 180 mg 100s Container Label

Fexofenadine Hydrochloride Tablets, USP 180 mg 100s Carton Label
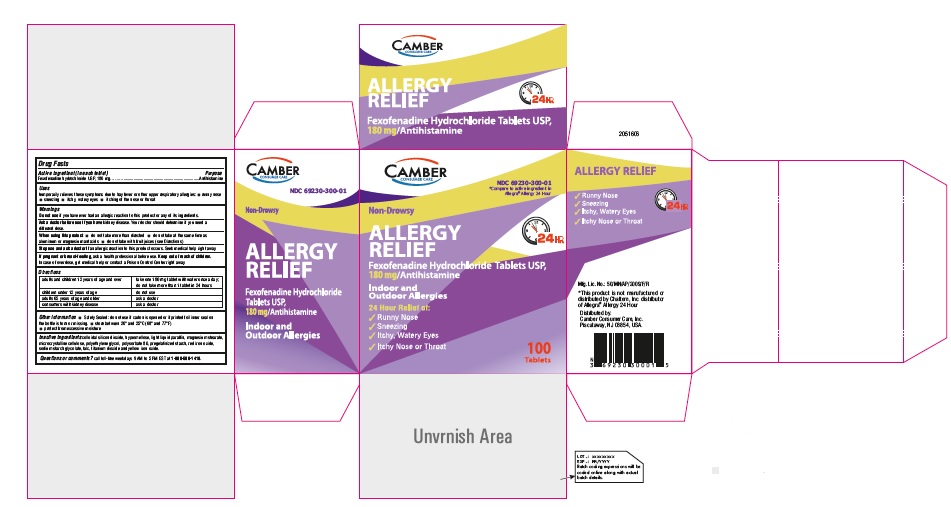
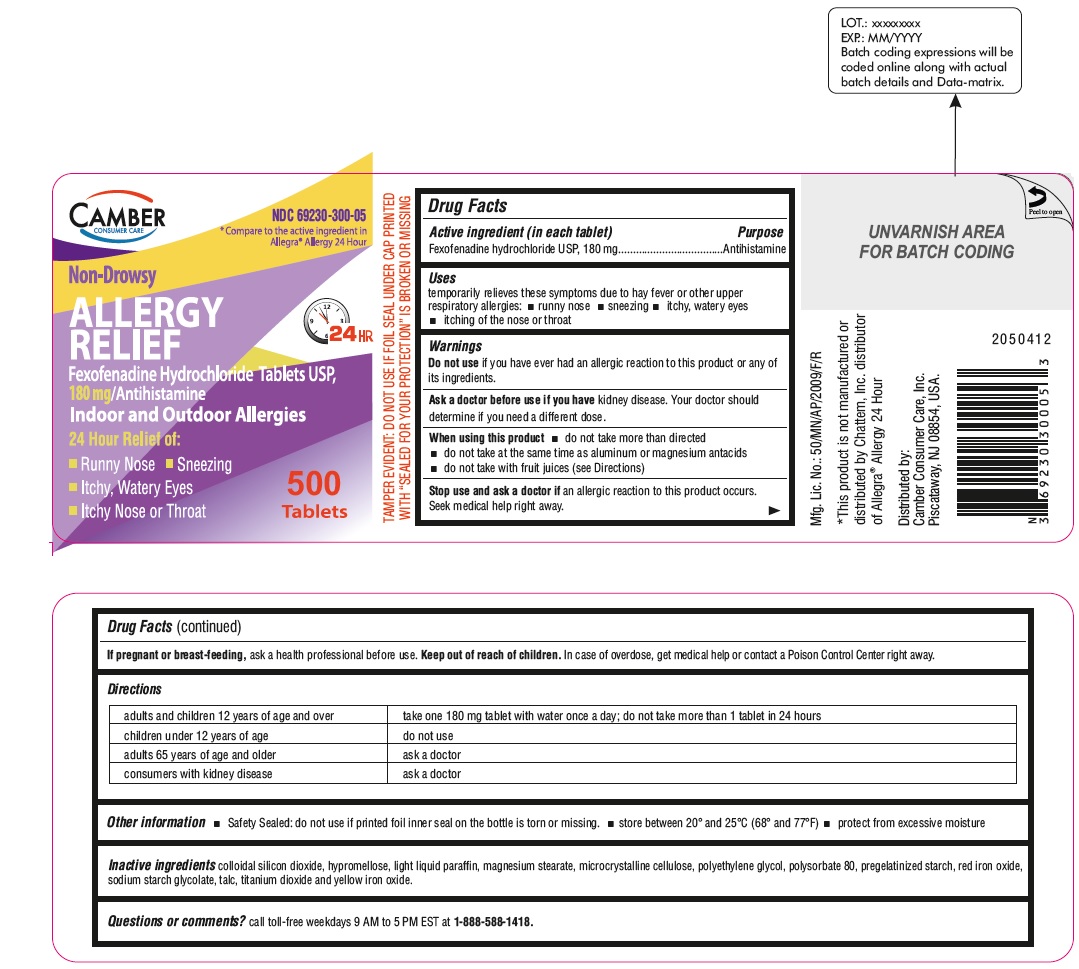
Fexofenadine Hydrochloride Tablets, USP 180 mg 500s Container Label
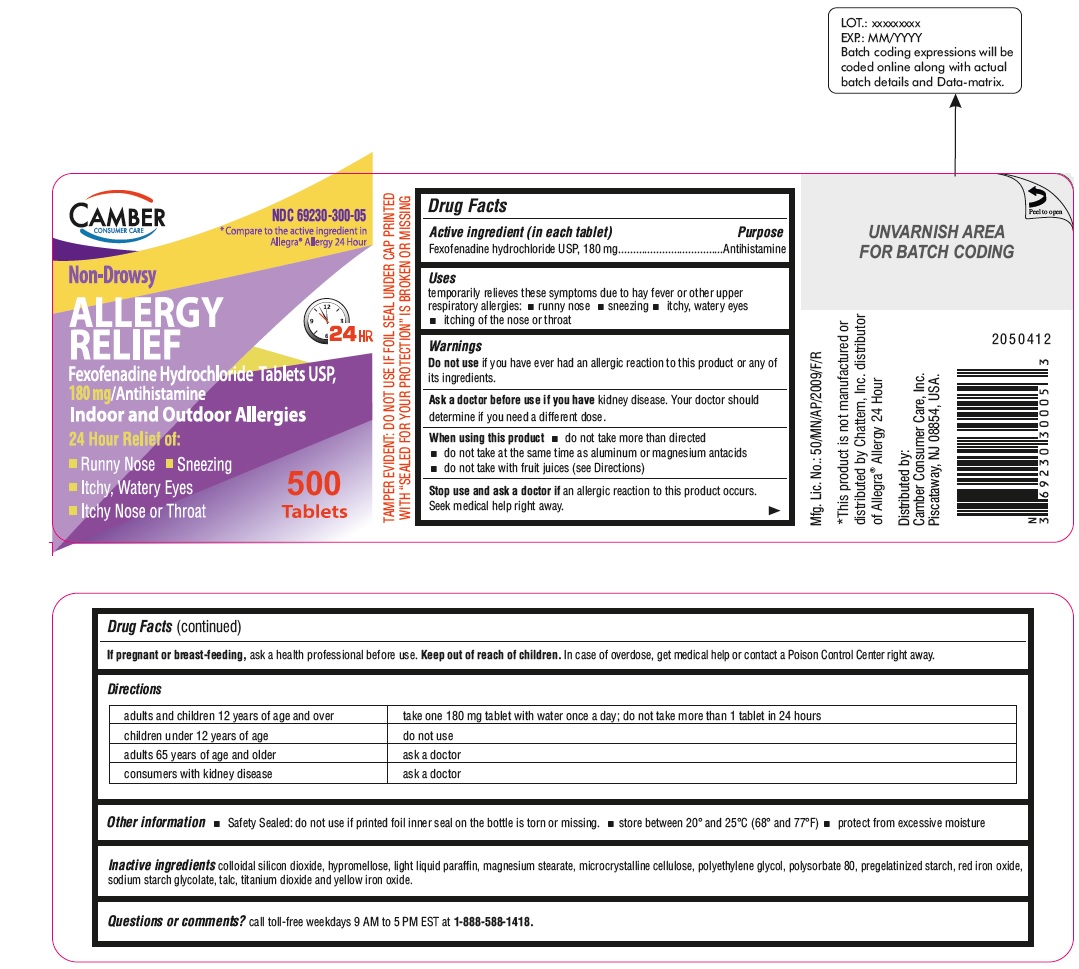
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HCL
fexofenadine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69230-300 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LIGHT MINERAL OIL (UNII: N6K5787QVP) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STARCH, CORN (UNII: O8232NY3SJ) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) Product Characteristics Color PINK Score no score Shape CAPSULE Size 18mm Flavor Imprint Code J;44 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69230-300-30 1 in 1 CARTON 08/19/2016 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:69230-300-01 1 in 1 CARTON 08/19/2016 2 100 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:69230-300-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/19/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204097 08/19/2016 FEXOFENADINE HCL
fexofenadine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69230-301 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LIGHT MINERAL OIL (UNII: N6K5787QVP) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STARCH, CORN (UNII: O8232NY3SJ) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) Product Characteristics Color PINK Score no score Shape OVAL Size 12mm Flavor Imprint Code J;43 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69230-301-30 1 in 1 CARTON 08/19/2016 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:69230-301-01 1 in 1 CARTON 08/19/2016 2 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204097 08/19/2016 Labeler - Camber Consumer Care (079539968) Establishment Name Address ID/FEI Business Operations Hetero Labs Limited Unit V 650452530 ANALYSIS(69230-300, 69230-301) , MANUFACTURE(69230-300, 69230-301)